High resolution structure of Streptavidin in complex with a novel high affinity peptide tag mimicking the biotin binding motif
Perbandt, M., Bruns, O., Vallazza, M., Lamla, T., Betzel, C., Erdmann, V.A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Streptavidin | 127 | Streptomyces avidinii | Mutation(s): 0 | 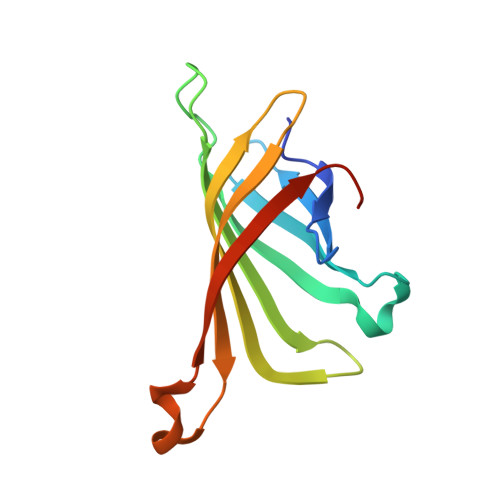 | |
UniProt | |||||
Find proteins for P22629 (Streptomyces avidinii) Explore P22629 Go to UniProtKB: P22629 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P22629 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| (FME)(ASP)(VAL)(GLU)(ALA)(TRP)(LEU) | C [auth X], D [auth Y] | 7 | N/A | Mutation(s): 0 | 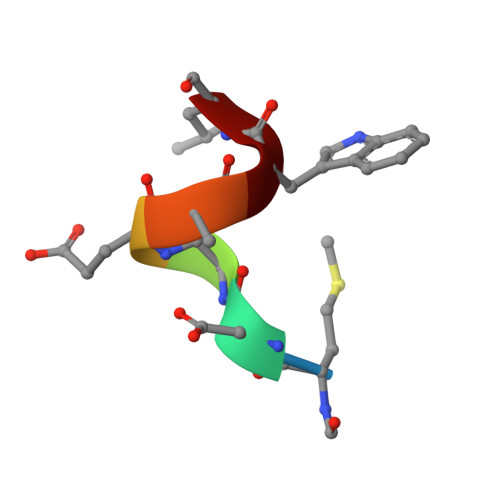 |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | E [auth A], G [auth B] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | F [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| FME Query on FME | C [auth X], D [auth Y] | L-PEPTIDE LINKING | C6 H11 N O3 S |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 80.488 | α = 90 |
| b = 81.563 | β = 90 |
| c = 85.749 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |