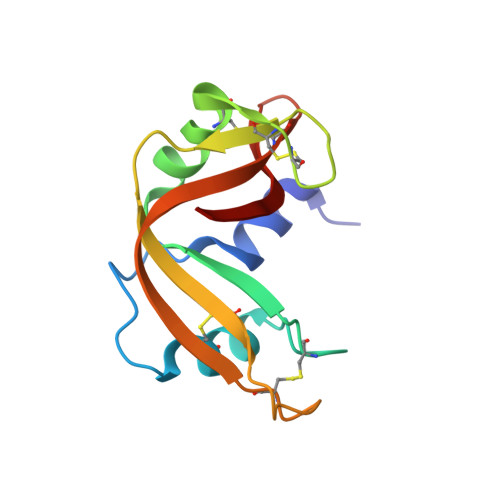
Characterization of an RNase with two catalytic centers. Human RNase6 catalytic and phosphate-binding site arrangement favors the endonuclease cleavage of polymeric substrates.
Prats-Ejarque, G., Blanco, J.A., Salazar, V.A., Nogues, V.M., Moussaoui, M., Boix, E.(2019) Biochim Biophys Acta Gen Subj 1863: 105-117
- PubMed: 30287244
- DOI: https://doi.org/10.1016/j.bbagen.2018.09.021
- Primary Citation of Related Structures:
5ET4, 5OAB, 5OGH, 6ENP - PubMed Abstract:
Human RNase6 is a small cationic antimicrobial protein that belongs to the vertebrate RNaseA superfamily. All members share a common catalytic mechanism, which involves a conserved catalytic triad, constituted by two histidines and a lysine (His15/His122/Lys38 in RNase6 corresponding to His12/His119/Lys41 in RNaseA). Recently, our first crystal structure of human RNase6 identified an additional His pair (His36/His39) and suggested the presence of a secondary active site.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Faculty of Biosciences, Universitat Autònoma de Barcelona, E-08193 Cerdanyola del Vallès, Spain. Electronic address: Guillem.Prats.Ejarque@uab.cat.