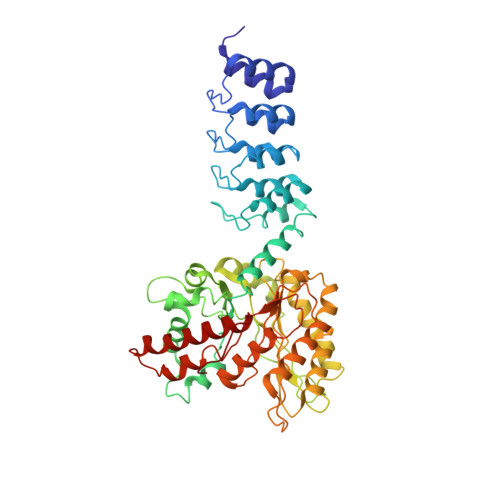
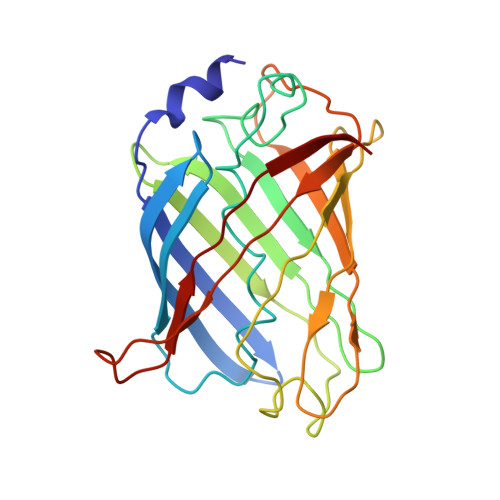
Fusion of DARPin to Aldolase Enables Visualization of Small Protein by Cryo-EM.
Yao, Q., Weaver, S.J., Mock, J.Y., Jensen, G.J.(2019) Structure 27: 1148
- PubMed: 31080120
- DOI: https://doi.org/10.1016/j.str.2019.04.003
- Primary Citation of Related Structures:
6MWQ - PubMed Abstract:
Solving protein structures by single-particle cryoelectron microscopy (cryo-EM) has become a crucial tool in structural biology. While exciting progress is being made toward the visualization of small macromolecules, the median protein size in both eukaryotes and bacteria is still beyond the reach of cryo-EM. To overcome this problem, we implemented a platform strategy in which a small protein target was rigidly attached to a large, symmetric base via a selectable adapter. Of our seven designs, the best construct used a designed ankyrin repeat protein (DARPin) rigidly fused to tetrameric rabbit muscle aldolase through a helical linker. The DARPin retained its ability to bind its target: GFP. We solved the structure of this complex to 3.0 Å resolution overall, with 5-8 Å resolution in the GFP region. As flexibility in the DARPin position limited the overall resolution of the target, we describe strategies to rigidify this element.
Organizational Affiliation:
Division of Biology and Biological Engineering, California Institute of Technology, 1200 E. California Boulevard, Pasadena, CA 91125, USA.