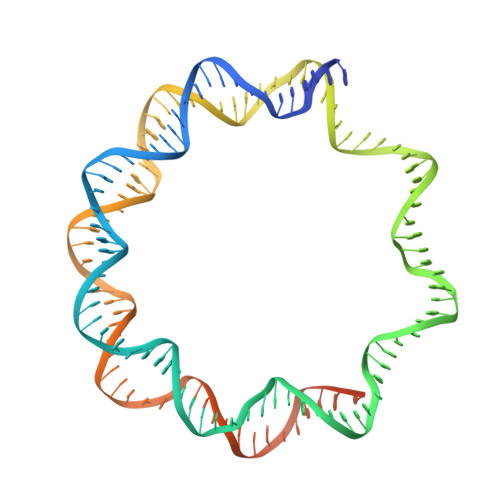
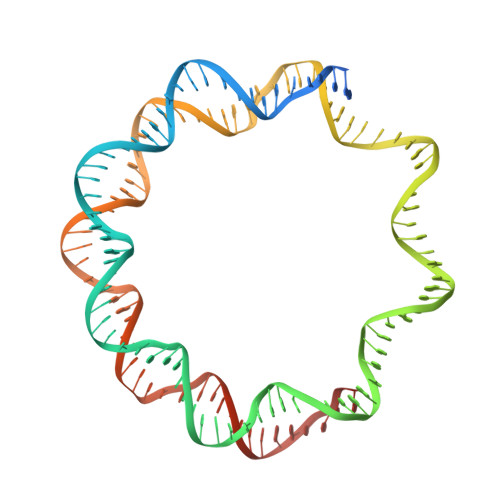
Phase-plate cryo-EM structure of the Widom 601 CENP-A nucleosome core particle reveals differential flexibility of the DNA ends.
Boopathi, R., Danev, R., Khoshouei, M., Kale, S., Nahata, S., Ramos, L., Angelov, D., Dimitrov, S., Hamiche, A., Petosa, C., Bednar, J.(2020) Nucleic Acids Res 48: 5735-5748
- PubMed: 32313946
- DOI: https://doi.org/10.1093/nar/gkaa246
- Primary Citation of Related Structures:
6TEM - PubMed Abstract:
The histone H3 variant CENP-A marks centromeres epigenetically and is essential for mitotic fidelity. Previous crystallographic studies of the CENP-A nucleosome core particle (NCP) reconstituted with a human α-satellite DNA derivative revealed both DNA ends to be highly flexible, a feature important for CENP-A mitotic functions. However, recent cryo-EM studies of CENP-A NCP complexes comprising primarily Widom 601 DNA reported well-ordered DNA ends. Here, we report the cryo-EM structure of the CENP-A 601 NCP determined by Volta phase-plate imaging. The data reveal that one ('left') 601 DNA end is well ordered whereas the other ('right') end is flexible and partly detached from the histone core, suggesting sequence-dependent dynamics of the DNA termini. Indeed, a molecular dynamics simulation of the CENP-A 601 NCP confirmed the distinct dynamics of the two DNA extremities. Reprocessing the image data using two-fold symmetry yielded a cryo-EM map in which both DNA ends appeared well ordered, indicating that such an artefact may inadvertently arise if NCP asymmetry is lost during image processing. These findings enhance our understanding of the dynamic features that discriminate CENP-A from H3 nucleosomes by revealing that DNA end flexibility can be fine-tuned in a sequence-dependent manner.
Organizational Affiliation:
Université Grenoble Alpes, CEA, CNRS, Institut de Biologie Structurale (IBS), 38000 Grenoble, France.