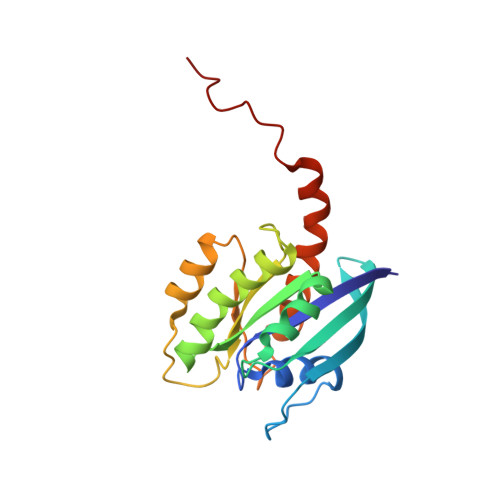
Two Distinct Structures of Membrane-Associated Homodimers of GTP- and GDP-Bound KRAS4B Revealed by Paramagnetic Relaxation Enhancement.
Lee, K.Y., Fang, Z., Enomoto, M., Gasmi-Seabrook, G., Zheng, L., Koide, S., Ikura, M., Marshall, C.B.(2020) Angew Chem Int Ed Engl 59: 11037-11045
- PubMed: 32227412
- DOI: https://doi.org/10.1002/anie.202001758
- Primary Citation of Related Structures:
6W4E, 6W4F - PubMed Abstract:
KRAS homo-dimerization has been implicated in the activation of RAF kinases, however, the mechanism and structural basis remain elusive. We developed a system to study KRAS dimerization on nanodiscs using paramagnetic relaxation enhancement (PRE) NMR spectroscopy, and determined distinct structures of membrane-anchored KRAS dimers in the active GTP- and inactive GDP-loaded states. Both dimerize through an α4-α5 interface, but the relative orientation of the protomers and their contacts differ substantially. Dimerization of KRAS-GTP, stabilized by electrostatic interactions between R135 and E168, favors an orientation on the membrane that promotes accessibility of the effector-binding site. Remarkably, "cross"-dimerization between GTP- and GDP-bound KRAS molecules is unfavorable. These models provide a platform to elucidate the structural basis of RAF activation by RAS and to develop inhibitors that can disrupt the KRAS dimerization. The methodology is applicable to many other farnesylated small GTPases.
Organizational Affiliation:
Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, M5G 1L7, Canada.