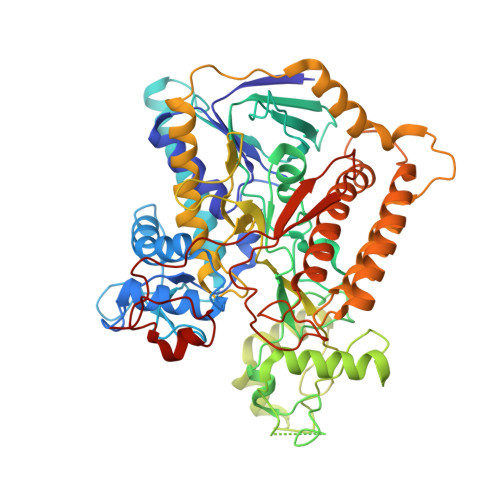
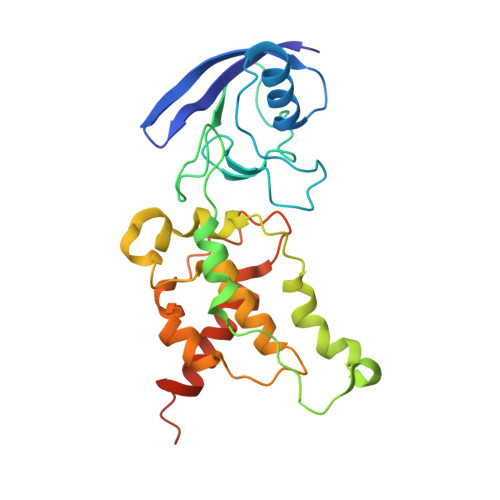
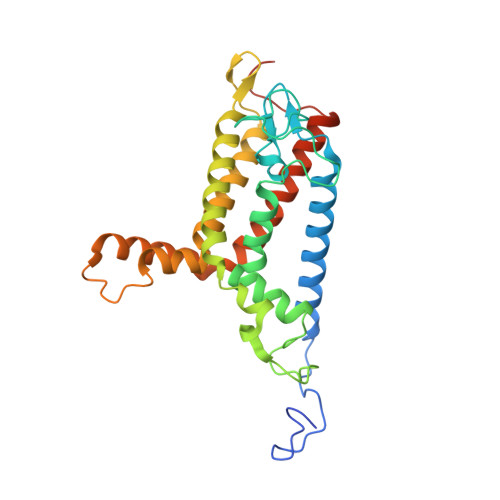
Architecture of the mycobacterial succinate dehydrogenase with a membrane-embedded Rieske FeS cluster.
Zhou, X., Gao, Y., Wang, W., Yang, X., Yang, X., Liu, F., Tang, Y., Lam, S.M., Shui, G., Yu, L., Tian, C., Guddat, L.W., Wang, Q., Rao, Z., Gong, H.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 33876763
- DOI: https://doi.org/10.1073/pnas.2022308118
- Primary Citation of Related Structures:
7D6V, 7D6X - PubMed Abstract:
Complex II, also known as succinate dehydrogenase (SQR) or fumarate reductase (QFR), is an enzyme involved in both the Krebs cycle and oxidative phosphorylation. Mycobacterial Sdh1 has recently been identified as a new class of respiratory complex II (type F) but with an unknown electron transfer mechanism. Here, using cryoelectron microscopy, we have determined the structure of Mycobacterium smegmatis Sdh1 in the presence and absence of the substrate, ubiquinone-1, at 2.53-Å and 2.88-Å resolution, respectively. Sdh1 comprises three subunits, two that are water soluble, SdhA and SdhB, and one that is membrane spanning, SdhC. Within these subunits we identified a quinone-binding site and a rarely observed Rieske-type [2Fe-2S] cluster, the latter being embedded in the transmembrane region. A mutant, where two His ligands of the Rieske-type [2Fe-2S] were changed to alanine, abolished the quinone reduction activity of the Sdh1. Our structures allow the proposal of an electron transfer pathway that connects the substrate-binding and quinone-binding sites. Given the unique features of Sdh1 and its essential role in Mycobacteria , these structures will facilitate antituberculosis drug discovery efforts that specifically target this complex.
Organizational Affiliation:
Shanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China.