1,4-Diazepane-2,5-diones as novel inhibitors of LFA-1
Wattanasin, S., Kallen, J., Myers, S., Guo, Q., Sabio, M., Ehrhardt, C., Albert, R., Hommel, U., Weckbecker, G., Welzenbach, K., Weitz-Schmidt, G.(2005) Bioorg Med Chem Lett 15: 1217-1220
- PubMed: 15686945
- DOI: https://doi.org/10.1016/j.bmcl.2004.11.072
- Primary Citation of Related Structures:
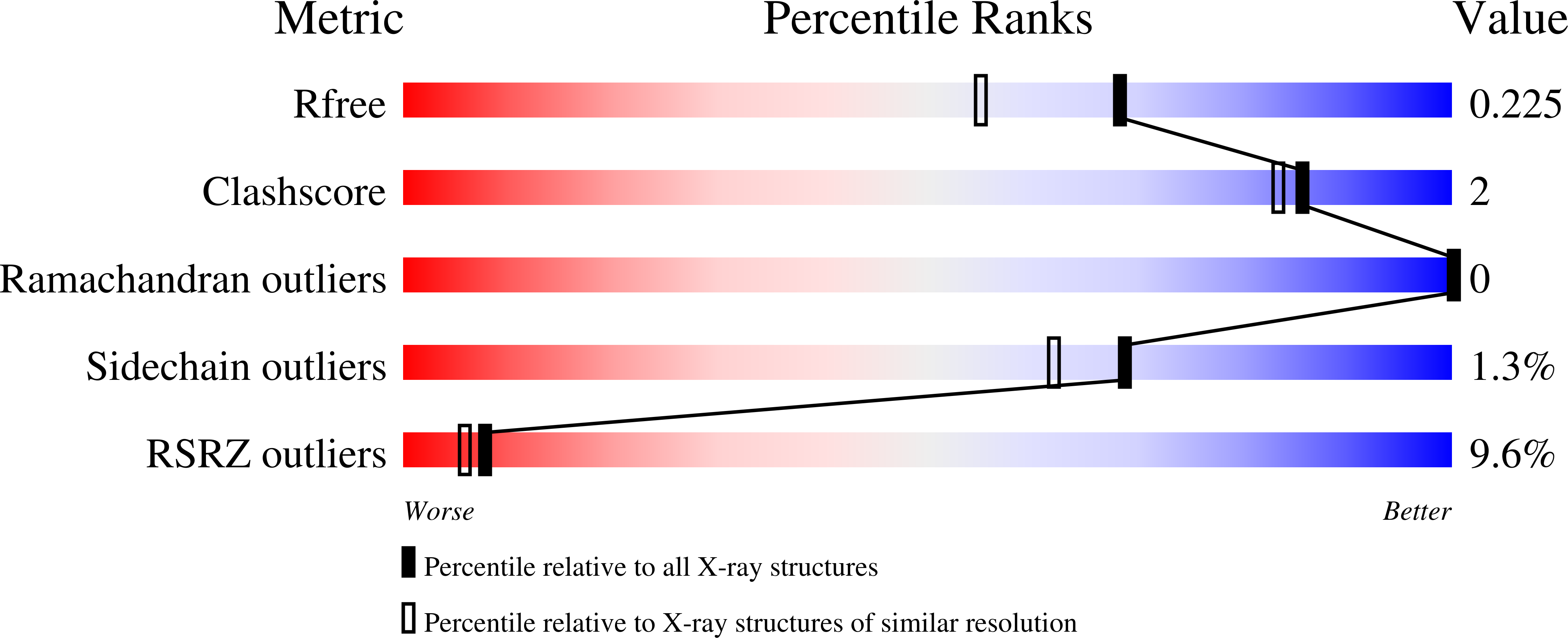
1XUO - PubMed Abstract:
1,4-Diazepane-2,5-diones (2) are found to be a new class of potent LFA-1 inhibitors. The synthesis, structure, and biological evaluation of these 1,4-diazepine-2,5-diones and related derivatives are described.
Organizational Affiliation:
Novartis Institutes for BioMedical Research, Cambridge, MA 02139, USA. sompong.wattanasin@pharma.novartis.com