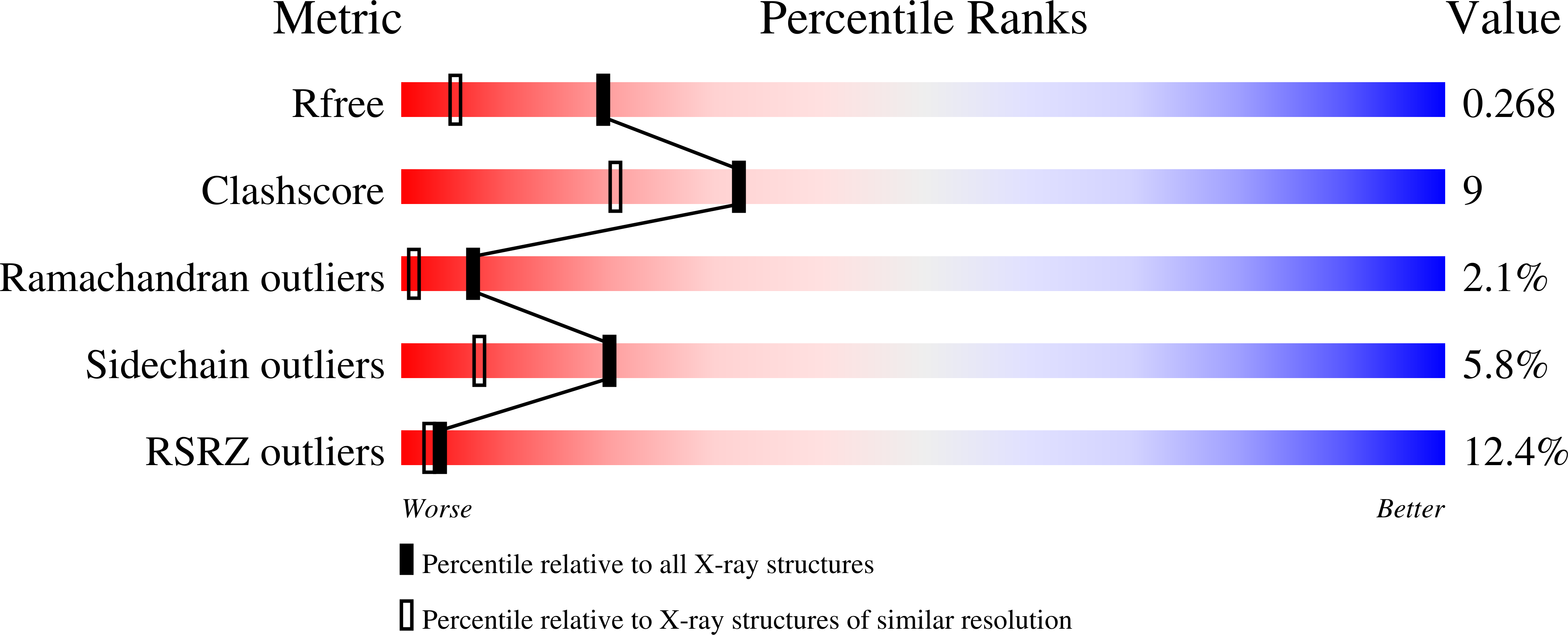
A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase.
Janjusevic, R., Abramovitch, R.B., Martin, G.B., Stebbins, C.E.(2006) Science 311: 222-226
- PubMed: 16373536
- DOI: https://doi.org/10.1126/science.1120131
- Primary Citation of Related Structures:
2FD4 - PubMed Abstract:
The Pseudomonas syringae protein AvrPtoB is translocated into plant cells, where it inhibits immunity-associated programmed cell death (PCD). The structure of a C-terminal domain of AvrPtoB that is essential for anti-PCD activity reveals an unexpected homology to the U-box and RING-finger components of eukaryotic E3 ubiquitin ligases, and we show that AvrPtoB has ubiquitin ligase activity. Mutation of conserved residues involved in the binding of E2 ubiquitin-conjugating enzymes abolishes this activity in vitro, as well as anti-PCD activity in tomato leaves, which dramatically decreases virulence. These results show that Pseudomonas syringae uses a mimic of host E3 ubiquitin ligases to inactivate plant defenses.
Organizational Affiliation:
Laboratory of Structural Microbiology, Rockefeller University, New York, NY 10021, USA.