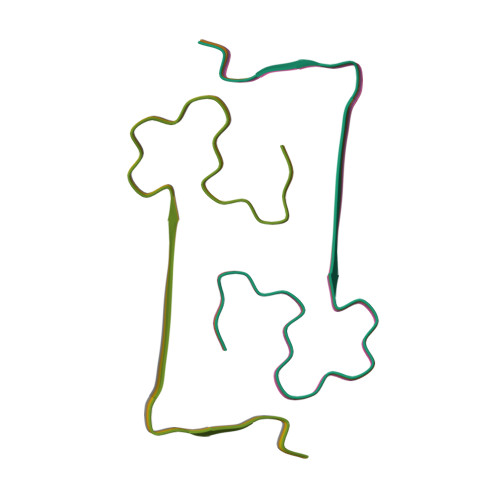
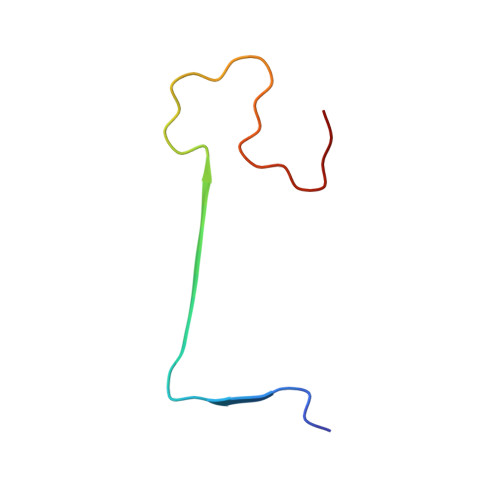
Atomic-Resolution Three-Dimensional Structure of Amyloid beta Fibrils Bearing the Osaka Mutation.
Schutz, A.K., Vagt, T., Huber, M., Ovchinnikova, O.Y., Cadalbert, R., Wall, J., Guntert, P., Bockmann, A., Glockshuber, R., Meier, B.H.(2015) Angew Chem Int Ed Engl 54: 331-335
- PubMed: 25395337
- DOI: https://doi.org/10.1002/anie.201408598
- Primary Citation of Related Structures:
2MVX - PubMed Abstract:
Despite its central importance for understanding the molecular basis of Alzheimer's disease (AD), high-resolution structural information on amyloid β-peptide (Aβ) fibrils, which are intimately linked with AD, is scarce. We report an atomic-resolution fibril structure of the Aβ1-40 peptide with the Osaka mutation (E22Δ), associated with early-onset AD. The structure, which differs substantially from all previously proposed models, is based on a large number of unambiguous intra- and intermolecular solid-state NMR distance restraints.
Organizational Affiliation:
Physical Chemistry, ETH Zürich, Vladimir-Prelog-Weg 2, 8093 Zurich (Switzerland).