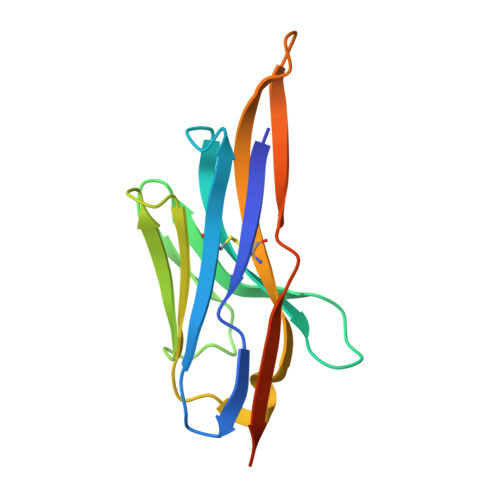
Structure of a low-melting-temperature anti-cholera toxin: llama V(H)H domain.
Legler, P.M., Zabetakis, D., Anderson, G.P., Lam, A., Hol, W.G., Goldman, E.R.(2013) Acta Crystallogr Sect F Struct Biol Cryst Commun 69: 90-93
- PubMed: 23385744
- DOI: https://doi.org/10.1107/S1744309112050750
- Primary Citation of Related Structures:
4IDL - PubMed Abstract:
Variable heavy domains derived from the heavy-chain-only antibodies found in camelids (V(H)H domains) are known for their thermal stability. Here, the structure of A9, an anti-cholera toxin V(H)H domain (K(d) = 77 ± 5 nM) that has an unusually low melting temperature of 319.9 ± 1.6 K, is reported. The CDR3 residues of A9 form a β-hairpin that is directed away from the former V(H)-V(L) interfacial surface, exposing hydrophobic residues to the solvent. A DALI structural similarity search showed that this CDR3 conformation is uncommon.
Organizational Affiliation:
Center for Bio/Molecular Science and Engineering, Naval Research Laboratory, Washington, DC 20375, USA. patricia.legler@nrl.navy.mil