Vaccine-elicited primate antibodies use a distinct approach to the HIV-1 primary receptor binding site informing vaccine redesign.
Tran, K., Poulsen, C., Guenaga, J., de Val Alda, N., Wilson, R., Sundling, C., Li, Y., Stanfield, R.L., Wilson, I.A., Ward, A.B., Karlsson Hedestam, G.B., Wyatt, R.T.(2014) Proc Natl Acad Sci U S A 111: E738-E747
- PubMed: 24550318
- DOI: https://doi.org/10.1073/pnas.1319512111
- Primary Citation of Related Structures:
4KTD, 4KTE - PubMed Abstract:
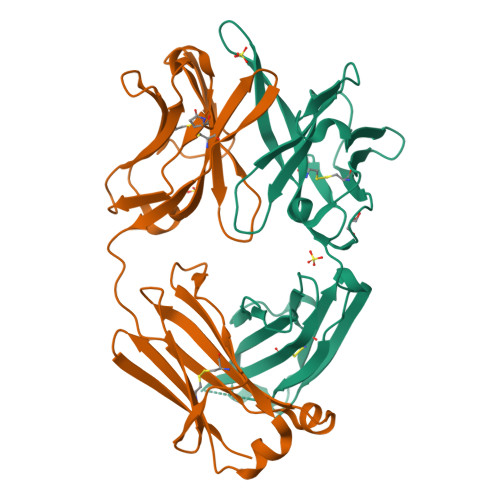
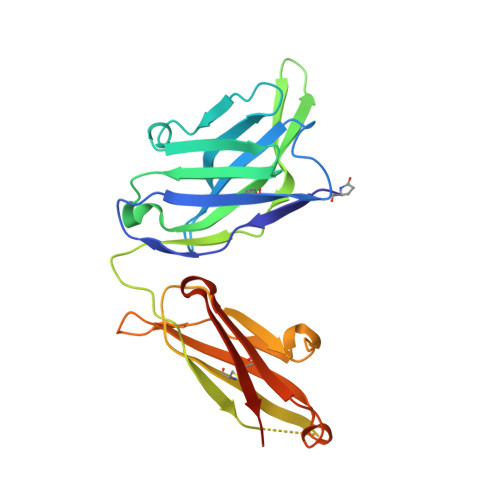
HIV-1 neutralization requires Ab accessibility to the functional envelope glycoprotein (Env) spike. We recently reported the isolation of previously unidentified vaccine-elicited, CD4 binding site (CD4bs)-directed mAbs from rhesus macaques immunized with soluble Env trimers, indicating that this region is immunogenic in the context of subunit vaccination. To elucidate the interaction of the trimer-elicited mAbs with gp120 and their insufficient interaction with the HIV-1 primary isolate spike, we crystallized the Fab fragments of two mAbs, GE136 and GE148. Alanine scanning of their complementarity-determining regions, coupled with epitope scanning of their epitopes on gp120, revealed putative contact residues at the Ab/gp120 interface. Docking of the GE136 and GE148 Fabs to gp120, coupled with EM reconstructions of these nonbroadly neutralizing mAbs (non-bNAbs) binding to gp120 monomers and EM modeling to well-ordered trimers, suggested Ab approach to the CD4bs by a vertical angle of access relative to the more lateral mode of interaction used by the CD4bs-directed bNAbs VRC01 and PGV04. Fitting the structures into the available cryo-EM native spike density indicated clashes between these two vaccine-elicited mAbs and the topside variable region spike cap, whereas the bNAbs duck under this quaternary shield to access the CD4bs effectively on primary HIV isolates. These results provide a structural basis for the limited neutralizing breadth observed by current vaccine-induced, CD4bs-directed Abs and highlight the need for better ordered trimer immunogens. The analysis presented here therefore provides valuable information to guide HIV-1 vaccine immunogen redesign.
Organizational Affiliation:
International AIDS Vaccine Initiative Neutralizing Antibody Center, Departments of Integrative Structural and Computational Biology and Immunology and Microbial Science, and Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery, The Scripps Research Institute, La Jolla, CA 92037.