Iripin-1, a new anti-inflammatory tick serpin, inhibits leukocyte recruitment in vivo while altering the levels of chemokines and adhesion molecules.
Chlastakova, A., Kascakova, B., Kotal, J., Langhansova, H., Kotsyfakis, M., Kuta Smatanova, I., Tirloni, L., Chmelar, J.(2023) Front Immunol 14: 1116324-1116324
- PubMed: 36756125
- DOI: https://doi.org/10.3389/fimmu.2023.1116324
- Primary Citation of Related Structures:
7QTZ - PubMed Abstract:
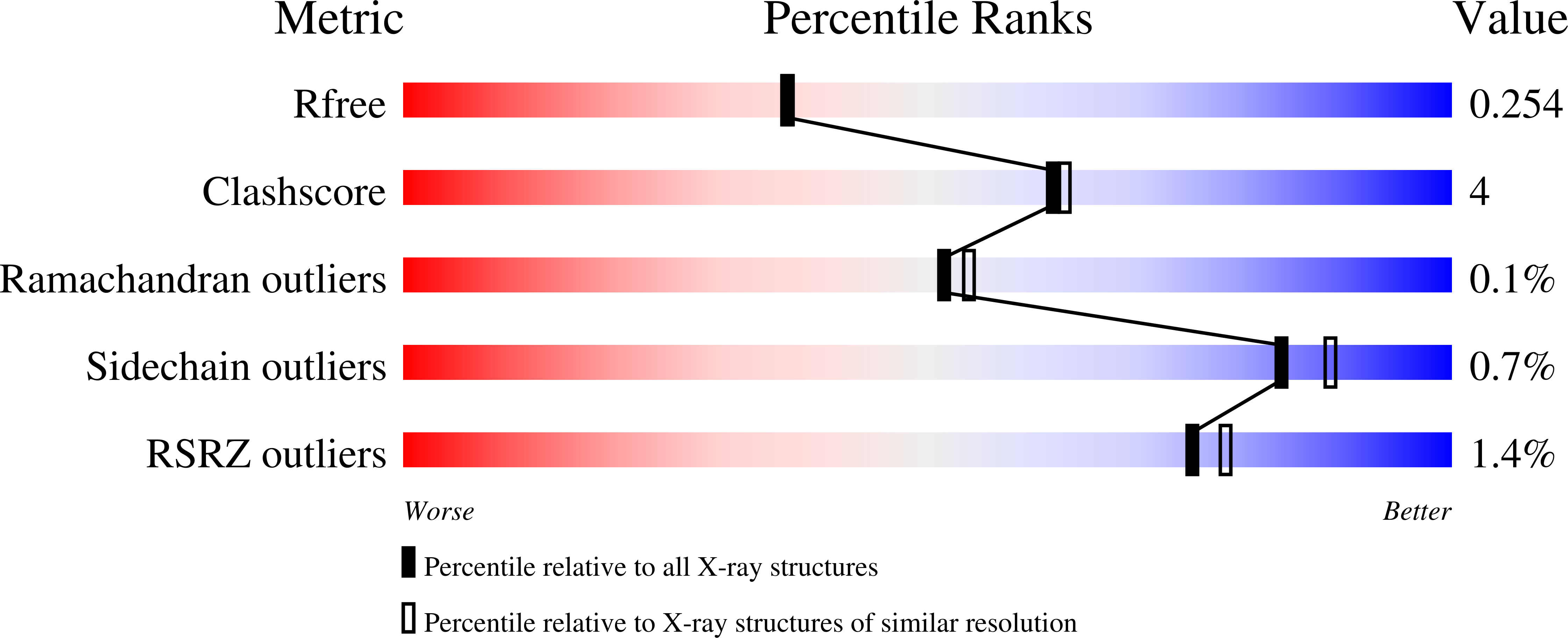
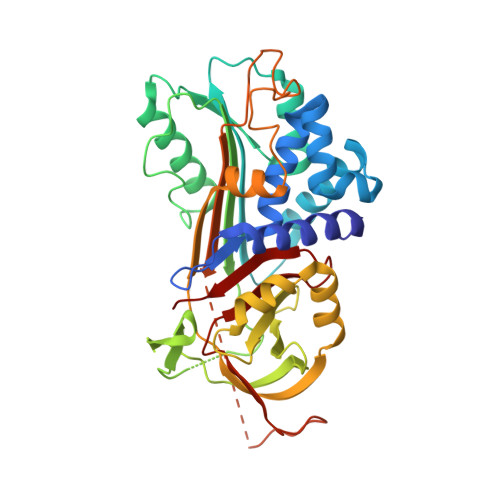
Serpins are widely distributed and functionally diverse inhibitors of serine proteases. Ticks secrete serpins with anti-coagulation, anti-inflammatory, and immunomodulatory activities via their saliva into the feeding cavity to modulate host's hemostatic and immune reaction initiated by the insertion of tick's mouthparts into skin. The suppression of the host's immune response not only allows ticks to feed on a host for several days but also creates favorable conditions for the transmission of tick-borne pathogens. Herein we present the functional and structural characterization of Iripin-1 ( Ixodes ricinus serpin-1), whose expression was detected in the salivary glands of the tick Ixodes ricinus , a European vector of tick-borne encephalitis and Lyme disease. Of 16 selected serine proteases, Iripin-1 inhibited primarily trypsin and further exhibited weaker inhibitory activity against kallikrein, matriptase, and plasmin. In the mouse model of acute peritonitis, Iripin-1 enhanced the production of the anti-inflammatory cytokine IL-10 and chemokines involved in neutrophil and monocyte recruitment, including MCP-1/CCL2, a potent histamine-releasing factor. Despite increased chemokine levels, the migration of neutrophils and monocytes to inflamed peritoneal cavities was significantly attenuated following Iripin-1 administration. Based on the results of in vitro experiments, immune cell recruitment might be inhibited due to Iripin-1-mediated reduction of the expression of chemokine receptors in neutrophils and adhesion molecules in endothelial cells. Decreased activity of serine proteases in the presence of Iripin-1 could further impede cell migration to the site of inflammation. Finally, we determined the tertiary structure of native Iripin-1 at 2.10 Å resolution by employing the X-ray crystallography technique. In conclusion, our data indicate that Iripin-1 facilitates I. ricinus feeding by attenuating the host's inflammatory response at the tick attachment site.
Organizational Affiliation:
Department of Medical Biology, Faculty of Science, University of South Bohemia in České Budějovice, České Budějovice, Czechia.