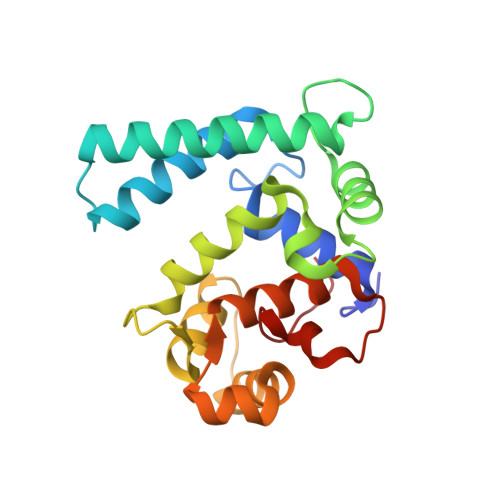
Crystal Structure Analysis of Sarcoplasmic-Calcium-Binding Protein: An Allergen in Scylla paramamosain.
Chen, Y., Jin, T., Li, M., Yun, X., Huan, F., Liu, Q., Hu, M., Wei, X., Zheng, P., Liu, G.(2023) J Agric Food Chem 71: 1214-1223
- PubMed: 36602420
- DOI: https://doi.org/10.1021/acs.jafc.2c07267
- Primary Citation of Related Structures:
7WBO - PubMed Abstract:
The structure of allergenic proteins provides important information about the binding of allergens to antibodies. In this study, the crystal structure of Scy p 4 with a resolution of 1.60 Å was obtained by X-ray diffraction. Epitope mapping of Scy p 4 revealed that linear epitopes are located on the surface of Scy p 4. Also, conformational epitopes are mostly located in the structural conservative region. Further structural comparison, surface electrostatic potential, and hydrogen bond force analysis showed that mutation of Asp 70 and Asp 18/20/70 would lead to calcium-binding capacity being lost and destruction of allergenicity. Furthermore, a comparative analysis of structure showed that sarcoplasmic-calcium-binding protein (SCP) had high sequence, secondary, and spatial structural identity in crustaceans, which may be an important factor leading to cross-reactivity among crustaceans. The structure of Scy p 4 provides a template for epitope evaluation and localization of SCPs, which will help to reveal cross-reactivity among species.
Organizational Affiliation:
College of Ocean Food and Biological Engineering, Xiamen Key Laboratory of Marine Functional Food, Fujian Provincial Engineering Technology Research Center of Marine Functional Food, Fujian Collaborative Innovation Center for Exploitation and Utilization of Marine Biological Resources, Jimei University, Xiamen, Fujian 361021, China.