Discovery and Characterization of a Myxobacterial Lanthipeptide with Unique Biosynthetic Features and Anti-inflammatory Activity.
Wang, X., Chen, X., Wang, Z.J., Zhuang, M., Zhong, L., Fu, C., Garcia, R., Muller, R., Zhang, Y., Yan, J., Wu, D., Huo, L.(2023) J Am Chem Soc 145: 16924-16937
- PubMed: 37466996
- DOI: https://doi.org/10.1021/jacs.3c06014
- Primary Citation of Related Structures:
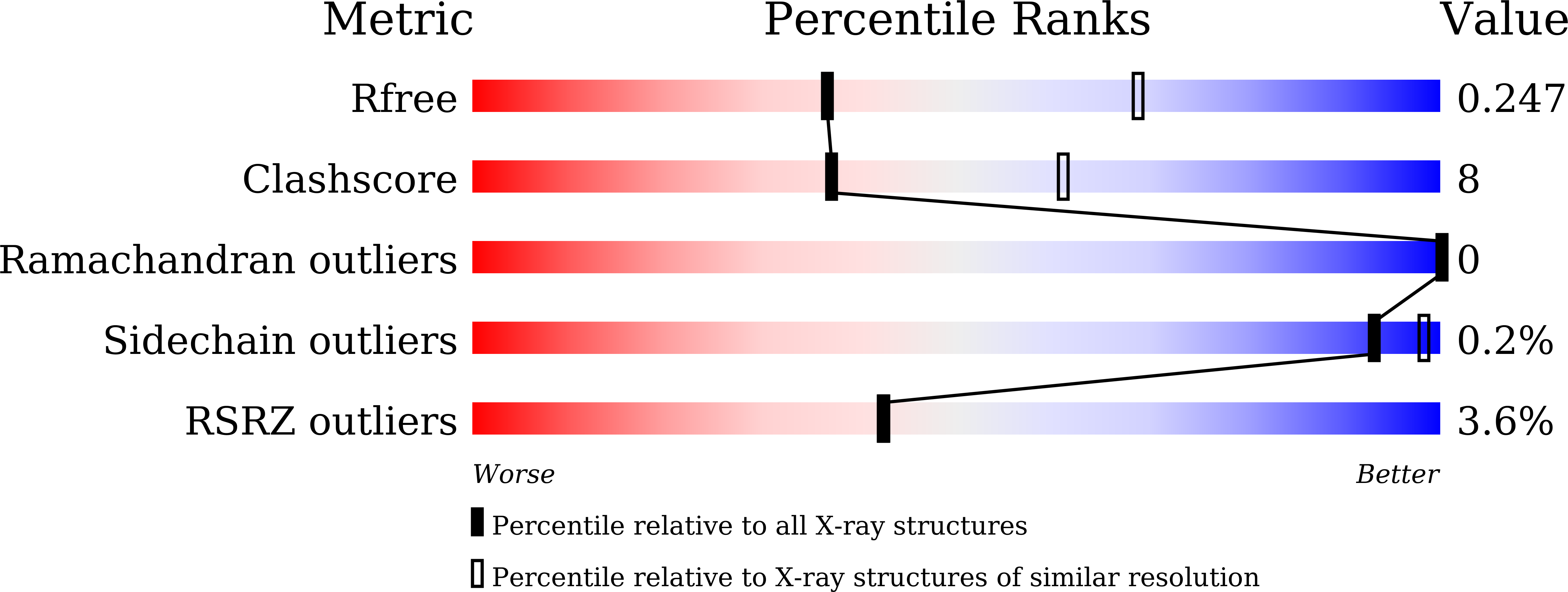
7XYO - PubMed Abstract:
The genomes of myxobacteria harbor a variety of biosynthetic gene clusters encoding numerous secondary metabolites, including ribosomally synthesized and post-translationally modified peptides (RiPPs) with diverse chemical structures and biological activities. However, the biosynthetic potential of RiPPs from myxobacteria remains barely explored. Herein, we report a novel myxobacteria lanthipeptide myxococin identified from Myxococcus fulvus . Myxococins represent the first example of lanthipeptides, of which the characteristic multiple thioether rings are installed by employing a Class II lanthipeptide synthetase MfuM and a Class I lanthipeptide cyclase MfuC in a cascaded way. Unprecedentedly, we biochemically characterized the first M61 family aminopeptidase MfuP involved in RiPP biosynthesis, demonstrating that MfuP showed the activity of an endopeptidase activity. MfuP is leader-independent but strictly selective for the multibridge structure of myxococin A and responsible for unwrapping two rings via amide bond hydrolysis, yielding myxococin B. Furthermore, the X-ray crystal structure of MfuP and structural analysis, including active-site mutations, are reported. Finally, myxococins are evaluated to exhibit anti-inflammatory activity in lipopolysaccharide-induced macrophages without detectable cytotoxicity.
Organizational Affiliation:
State Key Laboratory of Microbial Technology, Shandong University, Qingdao 266237, P. R. China.