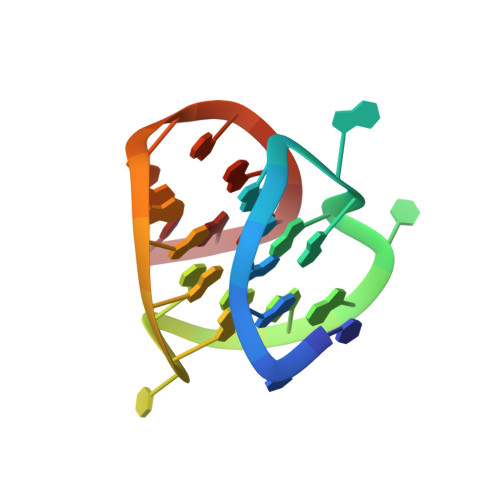
Structure of the Major G-Quadruplex in the Human EGFR Oncogene Promoter Adopts a Unique Folding Topology with a Distinctive Snap-Back Loop.
Liu, Y., Li, J., Zhang, Y., Wang, Y., Chen, J., Bian, Y., Xia, Y., Yang, M.H., Zheng, K., Wang, K.B., Kong, L.Y.(2023) J Am Chem Soc 145: 16228-16237
- PubMed: 37460135
- DOI: https://doi.org/10.1021/jacs.3c05214
- Primary Citation of Related Structures:
8JFQ - PubMed Abstract:
EGFR tyrosine kinase inhibitors have made remarkable success in targeted cancer therapy. However, therapeutic resistance inevitably occurred and EGFR-targeting therapy has been demonstrated to have limited efficacy or utility in glioblastoma, colorectal cancer, and hepatocellular carcinoma. Therefore, there is a high demand for the development of new targets to inhibit EGFR signaling. Herein, we found that the EGFR oncogene proximal promoter sequence forms a unique type of snap-back loop containing G-quadruplex (G4), which can be targeted by small molecules. For the first time, we determined the NMR solution structure of this snap-back EGFR -G4, a three-tetrad-core, parallel-stranded G4 with naturally occurring flanking residues at both the 5'-end and 3'-end. The snap-back loop located at the 3'-end region forms a stable capping structure through two stacked G-triads connected by multiple potential hydrogen bonds. Notably, the flanking residues are consistently absent in reported snap-back G4s, raising the question of whether such structures truly exist under in vivo conditions. The resolved EGFR -G4 structure has eliminated the doubt and showed distinct structural features that distinguish it from the previously reported snap-back G4s, which lack the flanking residues. Furthermore, we found that the snap-back EGFR -G4 structure is highly stable and can form on an elongated DNA template to inhibit DNA polymerase. The unprecedented high-resolution EGFR -G4 structure has thus contributed a promising molecular target for developing alternative EGFR signaling inhibitors in cancer therapeutics. Meanwhile, the two stacked triads may provide an attractive site for specific small-molecule targeting.
Organizational Affiliation:
Jiangsu Key Laboratory of Bioactive Natural Product Research and State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, Jiangsu 210009, People's Republic of China.