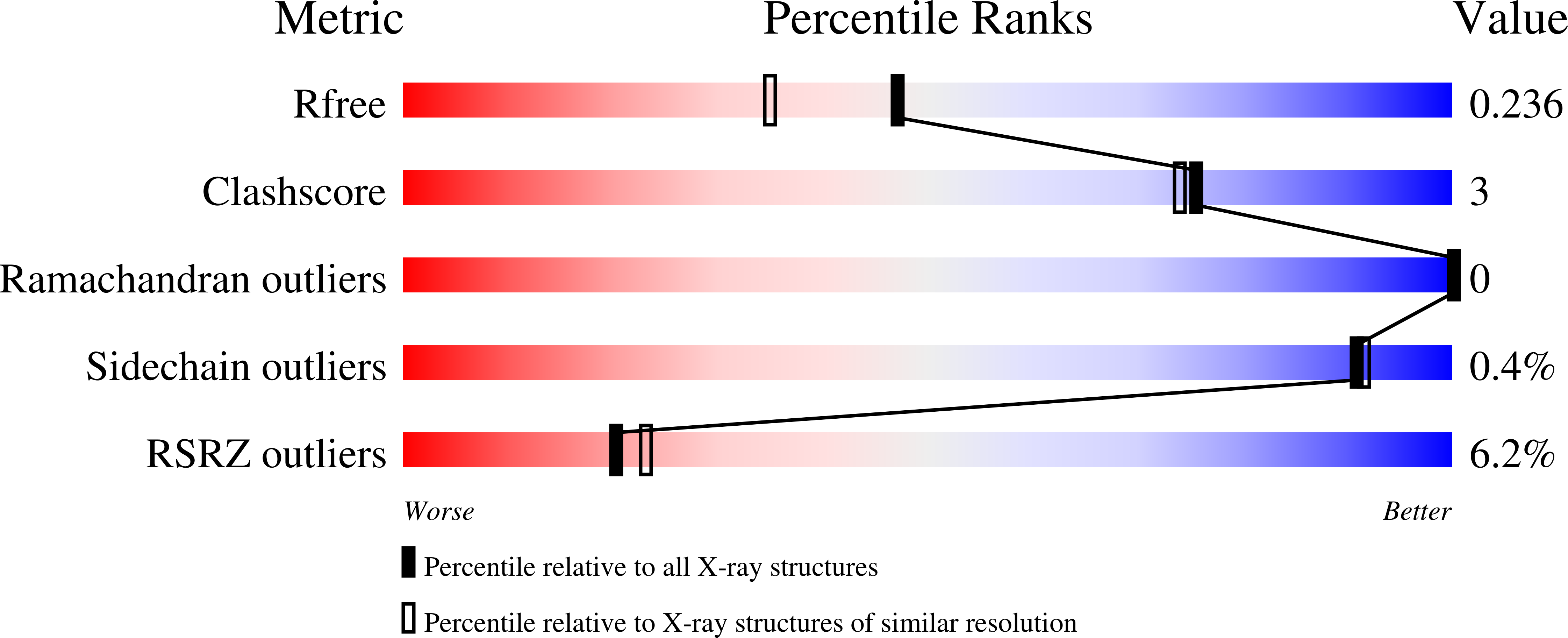
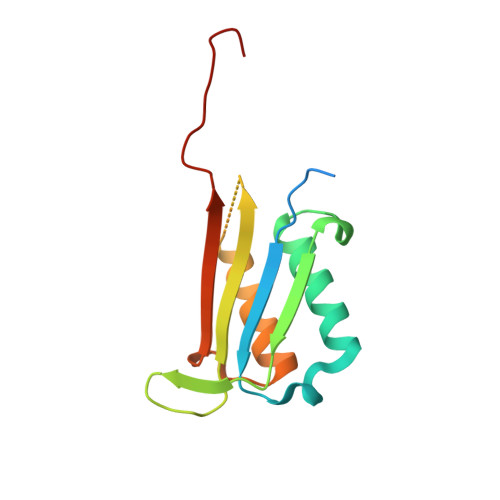
Crystal structure of N-terminally hexahistidine-tagged Onchocerca volvulus macrophage migration inhibitory factor-1.
Kimble, A.D., Dawson, O.C.O., Liu, L., Subramanian, S., Cooper, A., Battaile, K., Craig, J., Harmon, E., Myler, P., Lovell, S., Asojo, O.A.(2024) Acta Crystallogr F Struct Biol Commun 80: 328-334
- PubMed: 39503735
- DOI: https://doi.org/10.1107/S2053230X24010550
- Primary Citation of Related Structures:
8VJ2 - PubMed Abstract:
Onchocerca volvulus causes blindness, onchocerciasis, skin infections and devastating neurological diseases such as nodding syndrome. New treatments are needed because the currently used drug, ivermectin, is contraindicated in pregnant women and those co-infected with Loa loa. The Seattle Structural Genomics Center for Infectious Disease (SSGCID) produced, crystallized and determined the apo structure of N-terminally hexahistidine-tagged O. volvulus macrophage migration inhibitory factor-1 (His-OvMIF-1). OvMIF-1 is a possible drug target. His-OvMIF-1 has a unique jellyfish-like structure with a prototypical macrophage migration inhibitory factor (MIF) trimer as the `head' and a unique C-terminal `tail'. Deleting the N-terminal tag reveals an OvMIF-1 structure with a larger cavity than that observed in human MIF that can be targeted for drug repurposing and discovery. Removal of the tag will be necessary to determine the actual biological oligomer of OvMIF-1 because size-exclusion chomatographic analysis of His-OvMIF-1 suggests a monomer, while PISA analysis suggests a hexamer stabilized by the unique C-terminal tails.
Organizational Affiliation:
Department of Clinical Laboratory Science, College of Nursing and Allied Health Sciences, Howard University, 801 North Capitol Street, 4th Floor, Washington, DC 20002, USA.