Retrieving functional pathways of biomolecules from single-particle snapshots.
Dashti, A., Mashayekhi, G., Shekhar, M., Ben Hail, D., Salah, S., Schwander, P., des Georges, A., Singharoy, A., Frank, J., Ourmazd, A.(2020) Nat Commun 11: 4734-4734
- PubMed: 32948759
- DOI: https://doi.org/10.1038/s41467-020-18403-x
- Primary Citation of Related Structures:
6PV6, 7JMF, 7JMG, 7JMH, 7JMI, 7JMJ - PubMed Abstract:
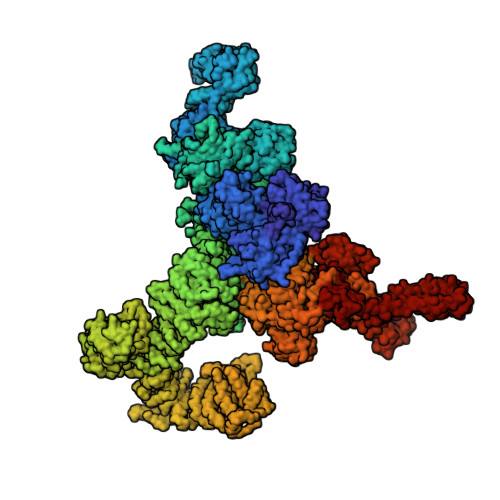
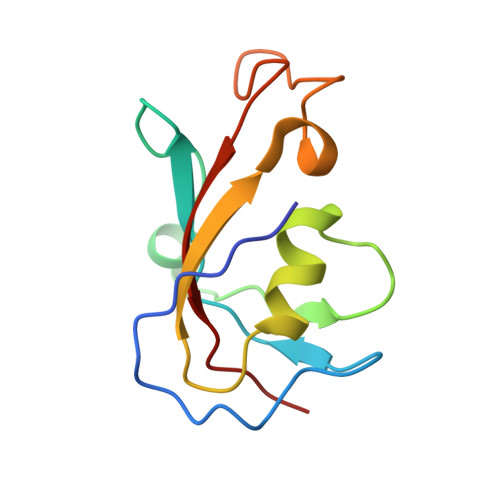
A primary reason for the intense interest in structural biology is the fact that knowledge of structure can elucidate macromolecular functions in living organisms. Sustained effort has resulted in an impressive arsenal of tools for determining the static structures. But under physiological conditions, macromolecules undergo continuous conformational changes, a subset of which are functionally important. Techniques for capturing the continuous conformational changes underlying function are essential for further progress. Here, we present chemically-detailed conformational movies of biological function, extracted data-analytically from experimental single-particle cryo-electron microscopy (cryo-EM) snapshots of ryanodine receptor type 1 (RyR1), a calcium-activated calcium channel engaged in the binding of ligands. The functional motions differ substantially from those inferred from static structures in the nature of conformationally active structural domains, the sequence and extent of conformational motions, and the way allosteric signals are transduced within and between domains. Our approach highlights the importance of combining experiment, advanced data analysis, and molecular simulations.
Organizational Affiliation:
Department of Physics, University of Wisconsin Milwaukee, 3135 N. Maryland Ave, Milwaukee, WI, 53211, USA.