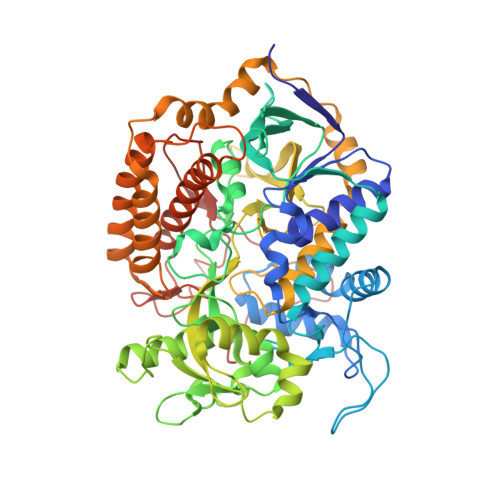
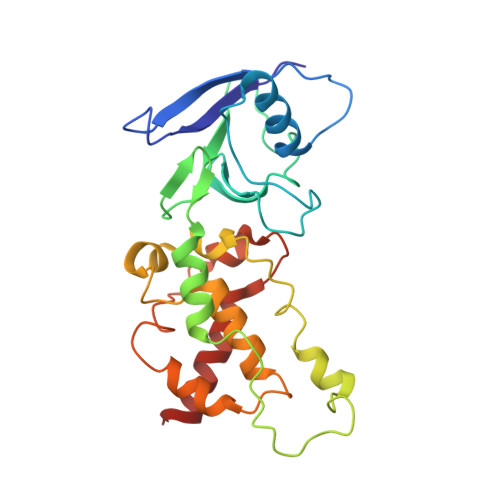
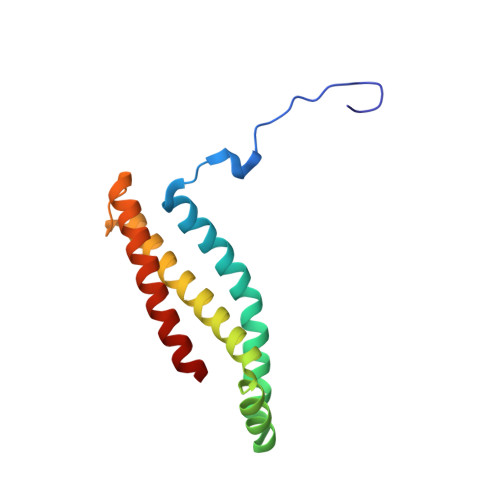
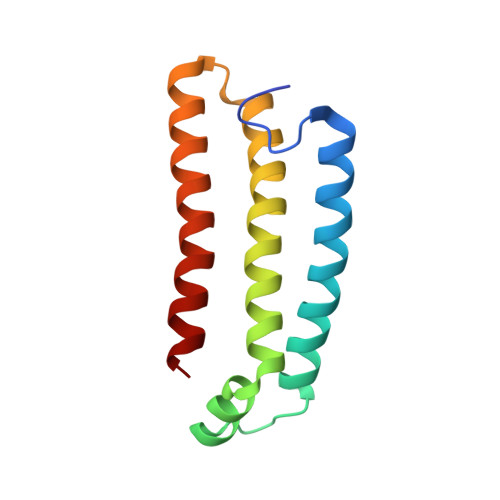
Architecture of succinate dehydrogenase and reactive oxygen species generation
Yankovskaya, V., Horsefield, R., Tornroth, S., Luna-Chavez, C., Miyoshi, H., Leger, C., Byrne, B., Cecchini, G., Iwata, S.(2003) Science 299: 700-704
- PubMed: 12560550
- DOI: https://doi.org/10.1126/science.1079605
- Primary Citation of Related Structures:
1NEK, 1NEN - PubMed Abstract:
The structure of Escherichia coli succinate dehydrogenase (SQR), analogous to the mitochondrial respiratory complex II, has been determined, revealing the electron transport pathway from the electron donor, succinate, to the terminal electron acceptor, ubiquinone. It was found that the SQR redox centers are arranged in a manner that aids the prevention of reactive oxygen species (ROS) formation at the flavin adenine dinucleotide. This is likely to be the main reason SQR is expressed during aerobic respiration rather than the related enzyme fumarate reductase, which produces high levels of ROS. Furthermore, symptoms of genetic disorders associated with mitochondrial SQR mutations may be a result of ROS formation resulting from impaired electron transport in the enzyme.
- Molecular Biology Division, VA Medical Center, San Francisco, CA 94121, USA.
Organizational Affiliation: