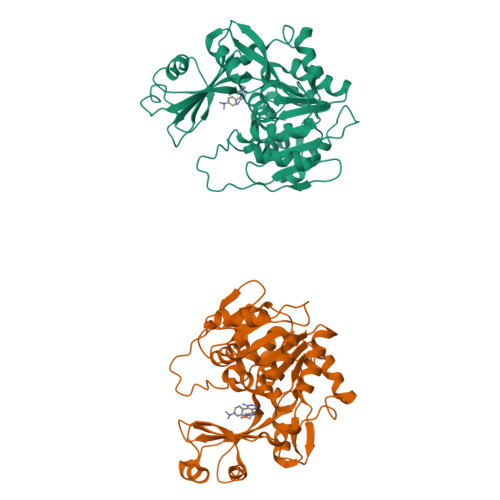
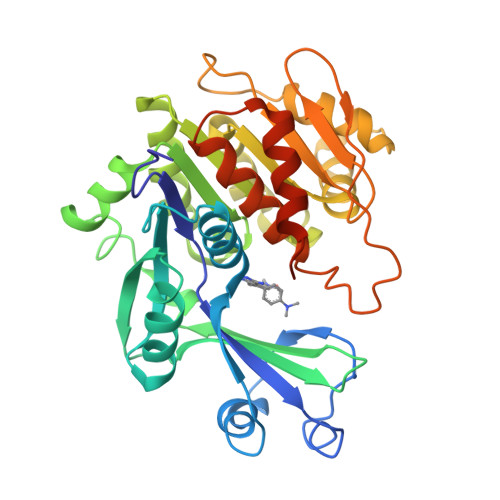
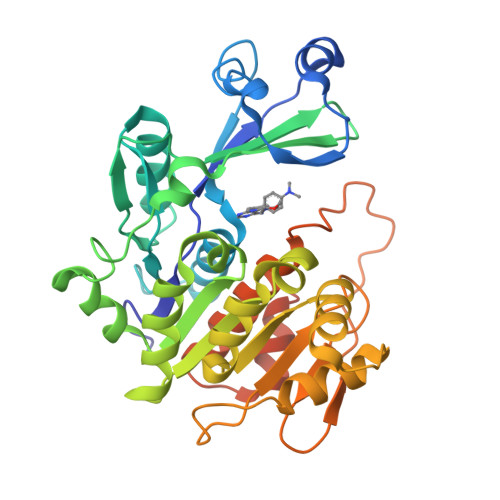
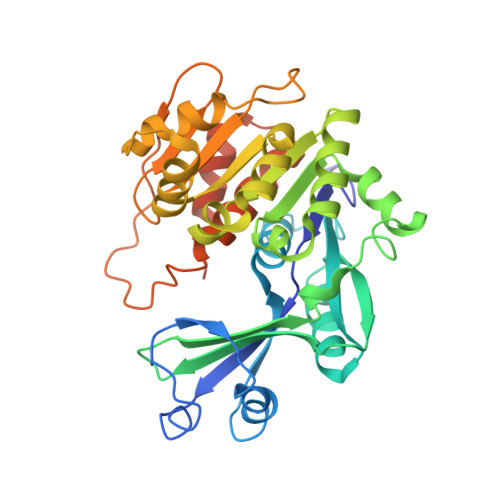
Crystal structures of human adenosine kinase inhibitor complexes reveal two distinct binding modes.
Muchmore, S.W., Smith, R.A., Stewart, A.O., Cowart, M.D., Gomtsyan, A., Matulenko, M.A., Yu, H., Severin, J.M., Bhagwat, S.S., Lee, C.H., Kowaluk, E.A., Jarvis, M.F., Jakob, C.L.(2006) J Med Chem 49: 6726-6731
- PubMed: 17154503
- DOI: https://doi.org/10.1021/jm060189a
- Primary Citation of Related Structures:
2I6A, 2I6B - PubMed Abstract:
Adenosine kinase (AK) is an enzyme responsible for converting endogenous adenosine (ADO) to adenosine monophosphate (AMP) in an adenosine triphosphate- (ATP-) dependent manner. The structure of AK consists of two domains, the first a large alpha/beta Rossmann-like nucleotide binding domain that forms the ATP binding site, and a smaller mixed alpha/beta domain, which, in combination with the larger domain, forms the ADO binding site and the site of phosphoryl transfer. AK inhibitors have been under investigation as antinociceptive, antiinflammatory, and anticonvulsant as well as antiinfective agents. In this work, we report the structures of AK in complex with two classes of inhibitors: the first, ADO-like, and the second, a novel alkynylpyrimidine series. The two classes of structures, which contain structurally similar substituents, reveal distinct binding modes in which the AK structure accommodates the inhibitor classes by a 30 degrees rotation of the small domain relative to the large domain. This change in binding mode stabilizes an open and a closed intermediate structural state and provide structural insight into the transition required for catalysis. This results in a significant rearrangement of both the protein active site and the orientation of the alkynylpyrimidine ligand when compared to the observed orientation of nucleosidic inhibitors or substrates.
Organizational Affiliation:
Structural Biology, R46Y, and Neuroscience Research, R4PM, Global Pharmaceutical Research and Development, Abbott Laboratories, 100 Abbott Park Road, Abbott Park, Illinois 60064, USA. steve.w.muchmore@abbott.com