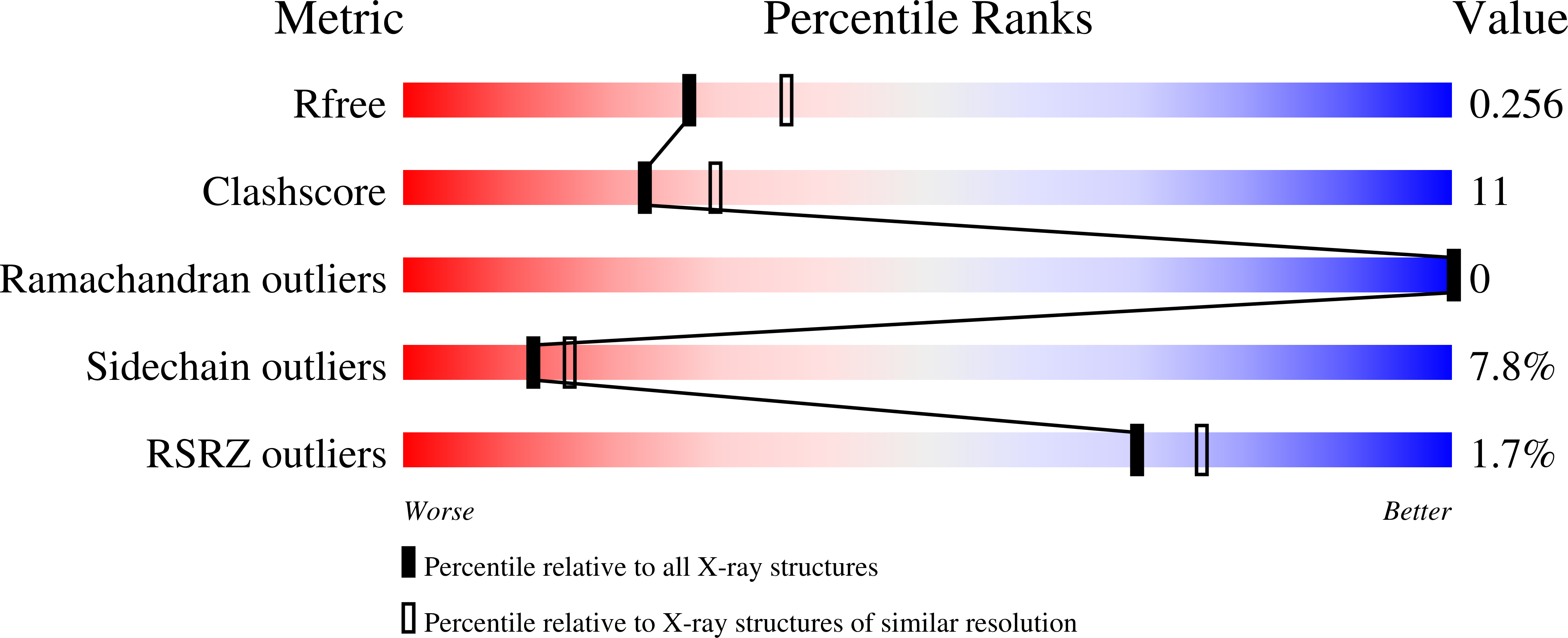
Structure-Based Protein Engineering Efforts with a Monomeric Tim Variant: The Importance of a Single Point Mutation for Generating an Active Site with Suitable Binding Properties.
Alahuhta, M., Salin, M., Casteleijn, M.G., Kemmer, C., El-Sayed, I., Augustyns, K., Neubauer, P., Wierenga, R.K.(2008) Protein Eng Des Sel 21: 257
- PubMed: 18239072
- DOI: https://doi.org/10.1093/protein/gzn002
- Primary Citation of Related Structures:
2VEI, 2VEK, 2VEL, 2VEM, 2VEN - PubMed Abstract:
A monomeric variant of triosephosphate isomerase (TIM) with a new engineered binding groove has been characterized further. In this variant (ml8bTIM), the phosphate binding loop had been shortened, causing the binding site to be much more extended. Here, it is reported that in the V233A variant of ml8bTIM (A-TIM), three important properties of the wild-type TIM active site have been restored: (i) the structural properties of loop-7, (ii) the binding site of a conserved water molecule between loop-7 and loop-8 and (iii) the binding site of the phosphate moiety. It is shown that the active site of A-TIM can bind TIM transition state analogs and suicide inhibitors competently. It is found that the active site geometry of the A-TIM complexes is less compact and more solvent exposed, as in wild-type TIM. This correlates with the observation that the catalytic efficiency of A-TIM for interconverting the TIM substrates is too low to be detected. It is also shown that the A-TIM active site can bind compounds which do not bind to wild-type TIM and which are completely different from the normal TIM substrate, like a citrate molecule. The binding of this citrate molecule is stabilized by hydrogen bonding interactions with the new binding groove.
Organizational Affiliation:
Biocenter Oulu, University of Oulu, Oulu, Finland..