Exploiting antigenic diversity for vaccine design: the Chlamydia ArtJ paradigm.
Soriani, M., Petit, P., Grifantini, R., Petracca, R., Gancitano, G., Frigimelica, E., Nardelli, F., Garcia, C., Spinelli, S., Scarabelli, G., Fiorucci, S., Affentranger, R., Ferrer-Navarro, M., Zacharias, M., Colombo, G., Vuillard, L., Daura, X., Grandi, G.(2010) J Biological Chem
- PubMed: 20592031
- DOI: https://doi.org/10.1074/jbc.M110.118513
- Primary Citation of Related Structures:
3DEL, 3N26 - PubMed Abstract:
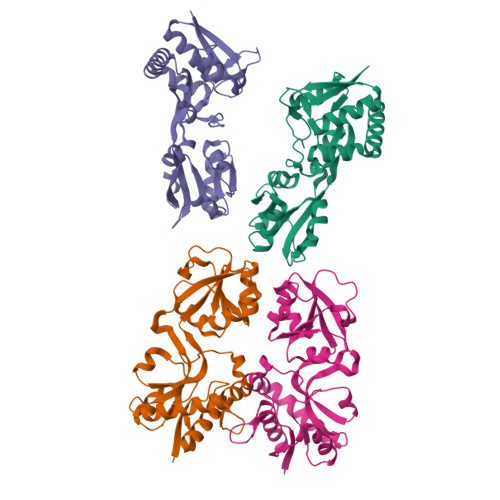
We present an interdisciplinary approach that, by incorporating a range of experimental and computational techniques, allows the identification and characterization of functional/immunogenic domains. This approach has been applied to ArtJ, an arginine-binding protein whose orthologs in Chlamydiae trachomatis (CT ArtJ) and pneumoniae (CPn ArtJ) are shown to have different immunogenic properties despite a high sequence similarity (60% identity). We have solved the crystallographic structures of CT ArtJ and CPn ArtJ, which are found to display a type II transporter fold organized in two α-β domains with the arginine-binding region at their interface. Although ArtJ is considered to belong to the periplasm, we found that both domains contain regions exposed on the bacterial surface. Moreover, we show that recombinant ArtJ binds to epithelial cells in vitro, suggesting a role for ArtJ in host-cell adhesion during Chlamydia infection. Experimental epitope mapping and computational analysis of physicochemical determinants of antibody recognition revealed that immunogenic epitopes reside mainly in the terminal (D1) domain of both CPn and CT ArtJ, whereas the surface properties of the respective binding-prone regions appear sufficiently different to assume divergent immunogenic behavior. Neutralization assays revealed that sera raised against CPn ArtJ D1 partially reduce both CPn and CT infectivity in vitro, suggesting that functional antibodies directed against this domain may potentially impair chlamydial infectivity. These findings suggest that the approach presented here, combining functional and structure-based analyses of evolutionary-related antigens can be a valuable tool for the identification of cross-species immunogenic epitopes for vaccine development.
Organizational Affiliation:
Novartis Vaccines, Via Fiorentina 1, 53100 Siena, Italy.