ATP and MO25alpha regulate the conformational state of the STRADalpha pseudokinase and activation of the LKB1 tumour suppressor
Zeqiraj, E., Filippi, B.M., Goldie, S., Navratilova, I., Boudeau, J., Deak, M., Alessi, D.R., van Aalten, D.M.(2009) PLoS Biol 7: e1000126-e1000126
- PubMed: 19513107
- DOI: https://doi.org/10.1371/journal.pbio.1000126
- Primary Citation of Related Structures:
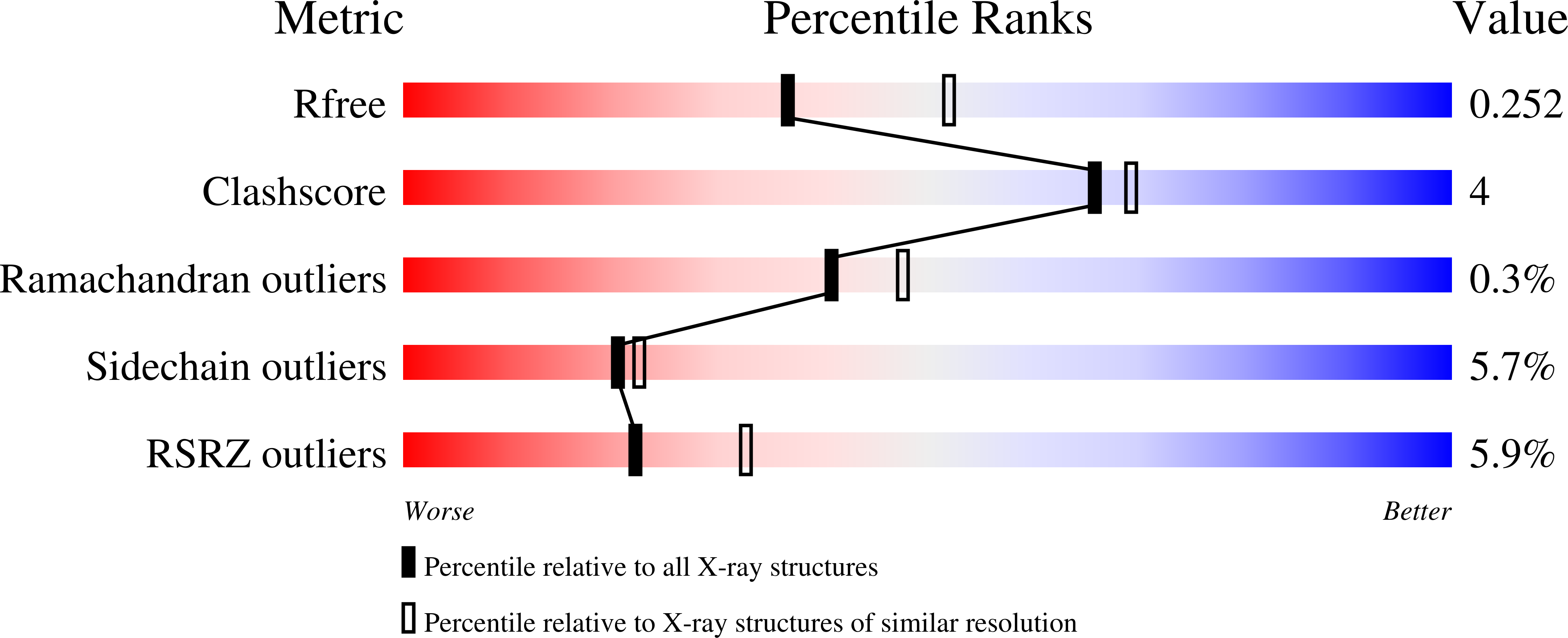
3GNI - PubMed Abstract:
Pseudokinases lack essential residues for kinase activity, yet are emerging as important regulators of signal transduction networks. The pseudokinase STRAD activates the LKB1 tumour suppressor by forming a heterotrimeric complex with LKB1 and the scaffolding protein MO25. Here, we describe the structure of STRADalpha in complex with MO25alpha. The structure reveals an intricate web of interactions between STRADalpha and MO25alpha involving the alphaC-helix of STRADalpha, reminiscent of the mechanism by which CDK2 interacts with cyclin A. Surprisingly, STRADalpha binds ATP and displays a closed conformation and an ordered activation loop, typical of active protein kinases. Inactivity is accounted for by nonconservative substitution of almost all essential catalytic residues. We demonstrate that binding of ATP enhances the affinity of STRADalpha for MO25alpha, and conversely, binding of MO25alpha promotes interaction of STRADalpha with ATP. Mutagenesis studies reveal that association of STRADalpha with either ATP or MO25alpha is essential for LKB1 activation. We conclude that ATP and MO25alpha cooperate to maintain STRADalpha in an "active" closed conformation required for LKB1 activation. It has recently been demonstrated that a mutation in human STRADalpha that truncates a C-terminal region of the pseudokinase domain leads to the polyhydramnios, megalencephaly, symptomatic epilepsy (PMSE) syndrome. We demonstrate this mutation destabilizes STRADalpha and prevents association with LKB1. In summary, our findings describe one of the first structures of a genuinely inactive pseudokinase. The ability of STRADalpha to activate LKB1 is dependent on a closed "active" conformation, aided by ATP and MO25alpha binding. Thus, the function of STRADalpha is mediated through an active kinase conformation rather than kinase activity. It is possible that other pseudokinases exert their function through nucleotide binding and active conformations.
Organizational Affiliation:
Division of Molecular Microbiology, College of Life Sciences, University of Dundee, Dundee, Scotland.