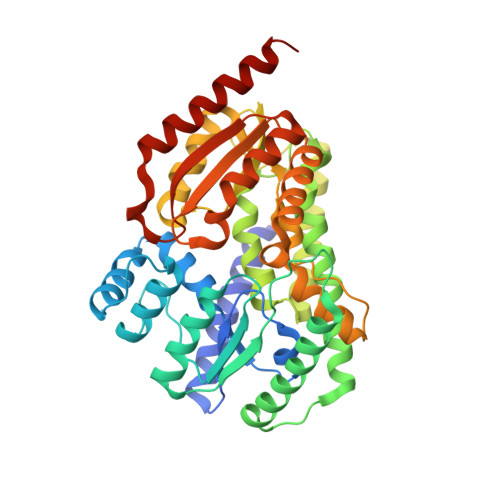
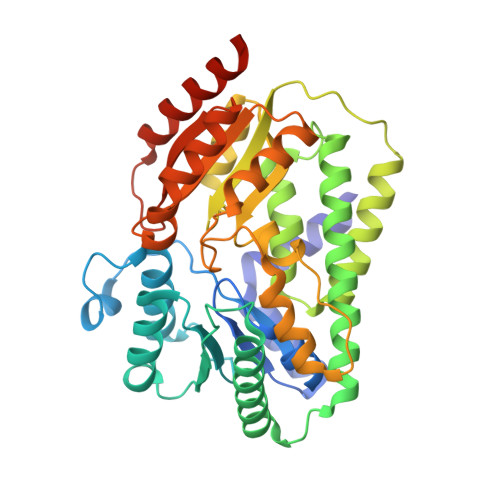
Structural Basis for Reductive Radical Formation and Electron Recycling in (R)-2-Hydroxyisocaproyl-CoA Dehydratase.
Knauer, S.H., Buckel, W., Dobbek, H.(2011) J Am Chem Soc 133: 4342-4347
- PubMed: 21366233
- DOI: https://doi.org/10.1021/ja1076537
- Primary Citation of Related Structures:
3O3M, 3O3N, 3O3O - PubMed Abstract:
The radical enzyme (R)-2-hydroxyisocaproyl-CoA dehydratase catalyzes the dehydration of (R)-2-hydroxyisocaproyl-CoA in the fermentation of l-leucine by the human pathogenic bacterium Clostridium difficile. In contrast to other radical enzymes, such as bacterial class II ribonucleotide reductase or biotin synthase, the Fe/S cluster containing (R)-2-hydroxyisocaproyl-CoA dehydratase requires no special cofactors such as coenzyme B(12) or S-adenosylmethionine for radical generation. Instead it uses a single high-energy electron that is recycled after each turnover. The catalyzed reaction, an atypical α/β-dehydration, depends on the reductive formation of ketyl radicals on the substrate generated by injection of a single electron from the ATP-dependent activator protein. So far, it is unknown how the active electron is recycled and how unwanted side reactions are prevented, allowing for up to 10,000 turnovers. The crystal structure reveals that the heterodimeric protein contains two [4Fe-4S] clusters at a distance of 12 Å, each coordinated by three cysteines and one terminal ligand. The cluster in the α-subunit is part of the active site. In the absence of substrate, a water/hydroxide ion acts as the fourth ligand. The substrate replaces this ligand and coordinates the cluster via the carbonyl-oxygen of the thioester group. The cluster in the β-subunit has a terminal sulfhydryl/sulfido ligand and can act as a reservoir to protect the electron from unwanted side reactions via a recycling mechanism. The crystal structure of (R)-2-hydroxyisocaproyl-CoA dehydratase serves as a model for the reductively radical-generating metalloenzymes of the (R)-2-hydroxyacyl-CoA dehydratase and benzoyl-CoA reductase families.
- Institut für Biologie, Strukturbiologie/Biochemie, Humboldt-Universität zu Berlin, D-10099 Berlin, Germany.
Organizational Affiliation: