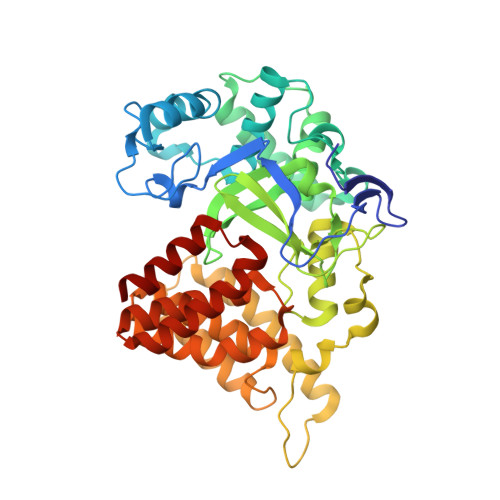
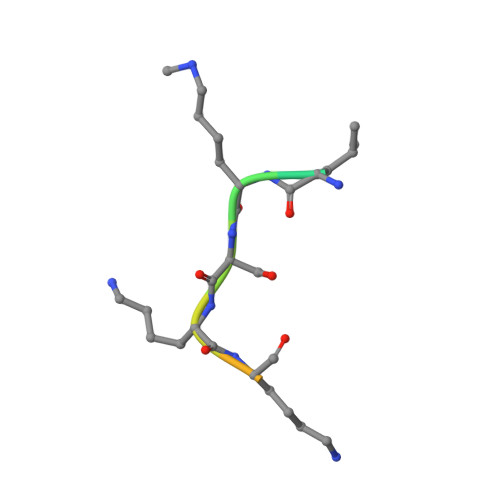
Structural Basis of Substrate Methylation and Inhibition of SMYD2.
Ferguson, A.D., Larsen, N.A., Howard, T., Pollard, H., Green, I., Grande, C., Cheung, T., Garcia-Arenas, R., Cowen, S., Wu, J., Godin, R., Chen, H., Keen, N.(2011) Structure 19: 1262-1273
- PubMed: 21782458
- DOI: https://doi.org/10.1016/j.str.2011.06.011
- Primary Citation of Related Structures:
3S7B, 3S7D, 3S7F, 3S7J - PubMed Abstract:
Protein lysine methyltransferases are important regulators of epigenetic signaling. These enzymes catalyze the transfer of donor methyl groups from S-adenosylmethionine to specific acceptor lysines on histones, leading to changes in chromatin structure and transcriptional regulation. These enzymes also methylate nonhistone protein substrates, revealing an additional mechanism to regulate cellular physiology. The oncogenic protein SMYD2 represses the functional activities of the tumor suppressor proteins p53 and Rb, making it an attractive drug target. Here we report the discovery of AZ505, a potent and selective inhibitor of SMYD2 that was identified from a high throughput chemical screen. We also present the crystal structures of SMYD2 with p53 substrate and product peptides, and notably, in complex with AZ505. This substrate competitive inhibitor is bound in the peptide binding groove of SMYD2. These results have implications for the development of SMYD2 inhibitors, and indicate the potential for developing novel therapies targeting this target class.
- DECS Structural Chemistry, AstraZeneca, Waltham, MA 02451, USA. andrew.ferguson@astrazeneca.com
Organizational Affiliation: