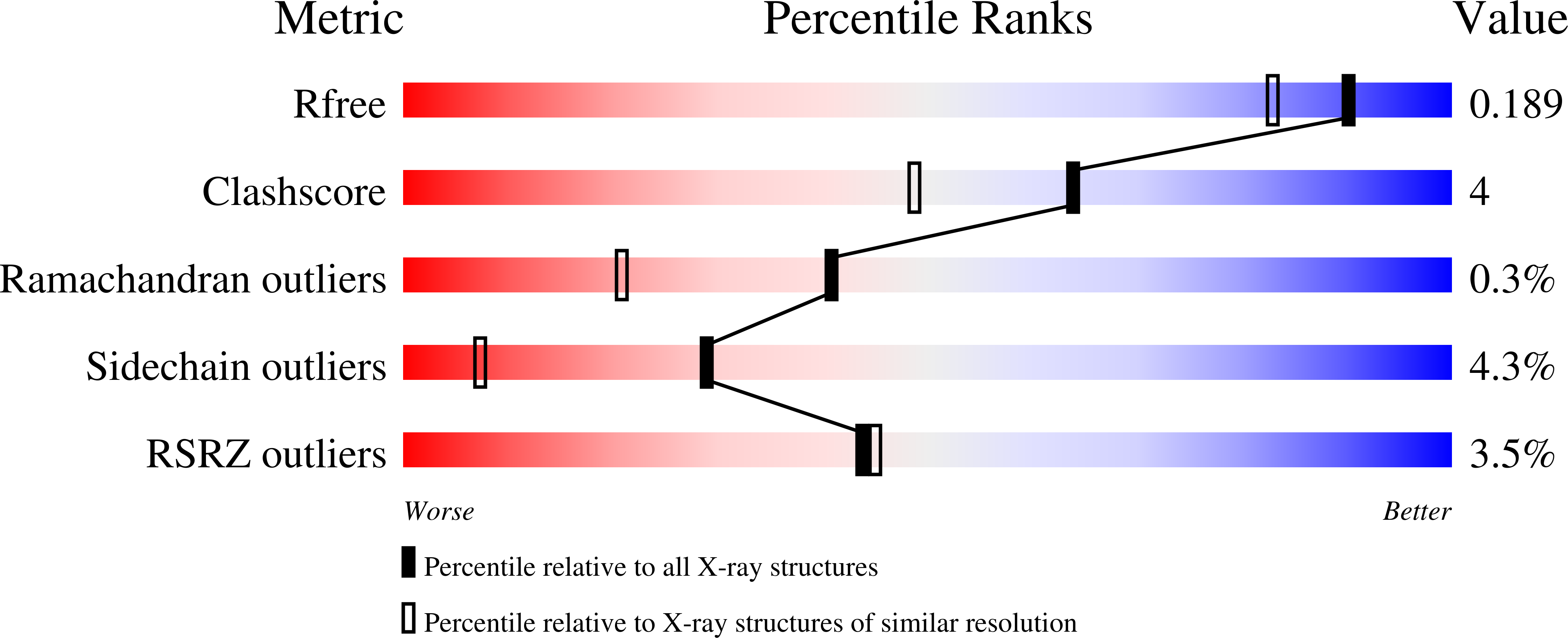
Mutation of Phe413 to Tyr in catalase KatE from Escherichia coli leads to side chain damage and main chain cleavage.
Jha, V., Donald, L.J., Loewen, P.C.(2012) Arch Biochem Biophys 525: 207-214
- PubMed: 22172685
- DOI: https://doi.org/10.1016/j.abb.2011.11.022
- Primary Citation of Related Structures:
3TTT, 3TTU, 3TTV, 3TTW, 3TTX - PubMed Abstract:
The monofunctional catalase KatE of Esherichia coli exhibits exceptional resistance to heat denaturation and proteolytic degradation. During an investigation of subtle conformation changes in Arg111 and Phe413 on the proximal side of the heme induced by H(2)O(2), variants at position R111, T115 and F413 were constructed. Because the residues are not situated in the distal side heme cavity where catalysis occurs, significant changes in reactivity were not expected and indeed, only small changes in the kinetic characteristics were observed in all of the variants. However, the F413Y variant was found to have undergone main chain cleavage whereas the R111A, T115A, F413E and F413K variants had not. Two sites of cleavage were identified in the crystal structure and by mass spectrometry at residues 111 and 115. In addition to main chain cleavage, modifications to the side chains of Tyr413, Thr115 and Arg111 were suggested by differences in the electron density maps compared to maps of the native and inactive variant H128N/F413Y. The inactive variant H128N/F413Y and the active variant T115A/F413Y both did not exhibit main chain cleavage and the R11A/F413Y variant exhibited less cleavage. In addition, the apparent modification of three side chains was largely absent in these variants. It is also significant that all three F413 single variants contained heme b suggesting that the fidelity of the phenyl group was important for mediating heme b oxidation to heme d. The reactions are attributed to the introduction of a new reactive center possibly involving a transient radical on Tyr413 formed during catalytic turn over.
Organizational Affiliation:
Department of Microbiology, University of Manitoba, Winnipeg, Canada MB R3T 2N2.