Fingerprinting Redox and Ligand States in Haemprotein Crystal Structures Using Resonance Raman Spectroscopy.
Kekilli, D., Dworkowski, F.S., Pompidor, G., Fuchs, M.R., Andrew, C.R., Antonyuk, S., Strange, R.W., Eady, R.R., Hasnain, S.S., Hough, M.A.(2014) Acta Crystallogr D Biol Crystallogr 70: 1289
- PubMed: 24816098
- DOI: https://doi.org/10.1107/S1399004714004039
- Primary Citation of Related Structures:
4CDA, 4CDV, 4CDY, 4CIP, 4CJG, 4CJO - PubMed Abstract:
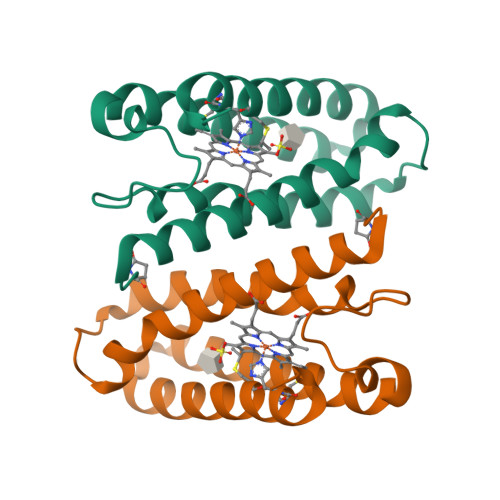
It is crucial to assign the correct redox and ligand states to crystal structures of proteins with an active redox centre to gain valid functional information and prevent the misinterpretation of structures. Single-crystal spectroscopies, particularly when applied in situ at macromolecular crystallography beamlines, allow spectroscopic investigations of redox and ligand states and the identification of reaction intermediates in protein crystals during the collection of structural data. Single-crystal resonance Raman spectroscopy was carried out in combination with macromolecular crystallography on Swiss Light Source beamline X10SA using cytochrome c' from Alcaligenes xylosoxidans. This allowed the fingerprinting and validation of different redox and ligand states, identification of vibrational modes and identification of intermediates together with monitoring of radiation-induced changes. This combined approach provides a powerful tool to obtain complementary data and correctly assign the true oxidation and ligand state(s) in redox-protein crystals.
Organizational Affiliation:
School of Biological Sciences, University of Essex, Wivenhoe Park, Colchester CO4 3SQ, England.