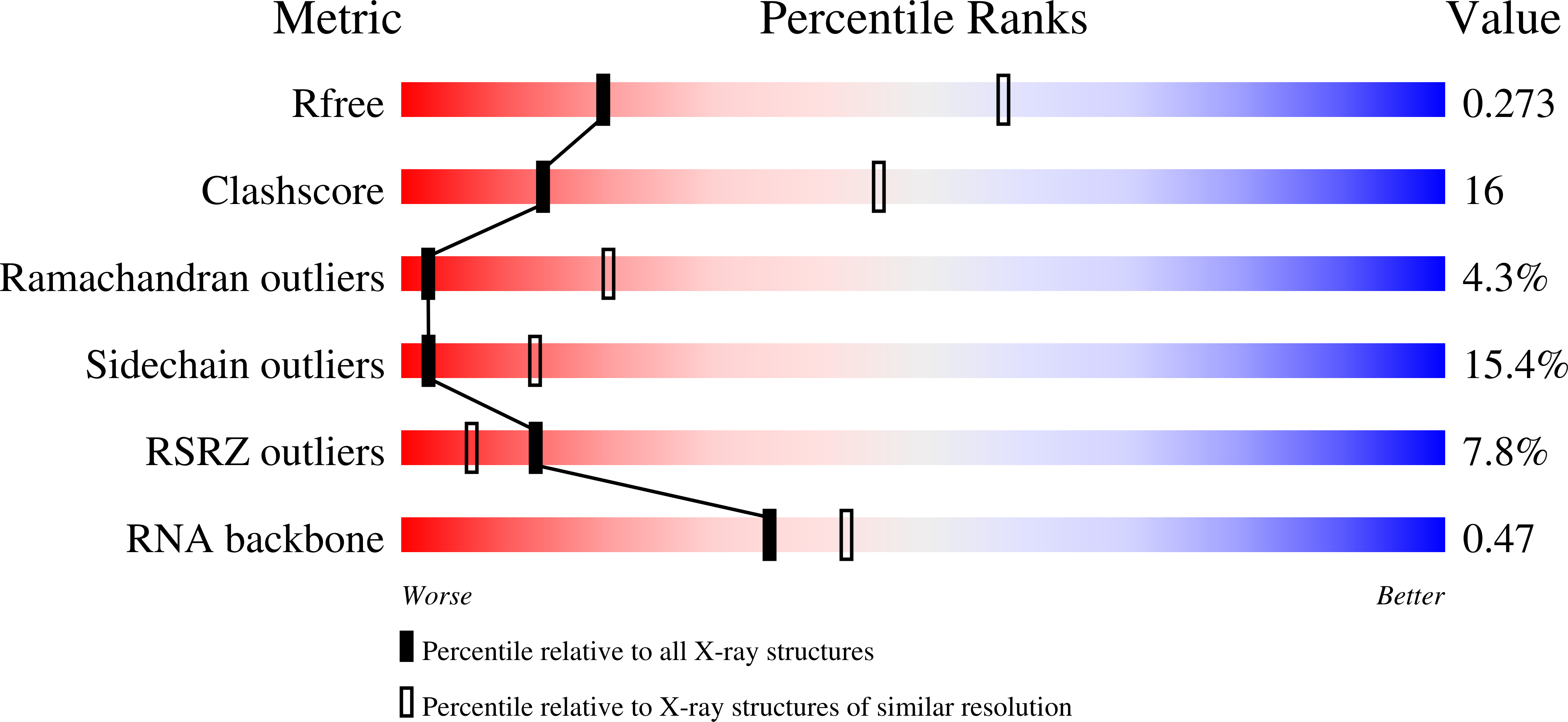
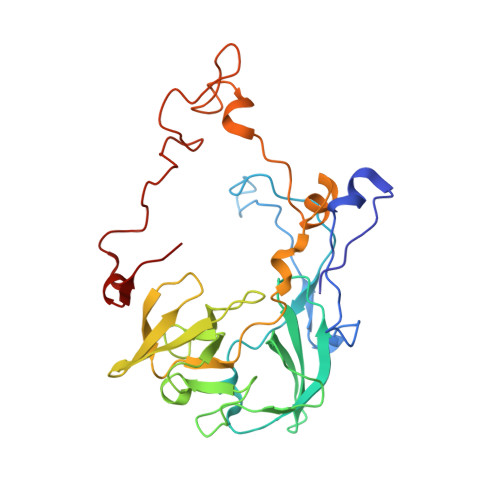
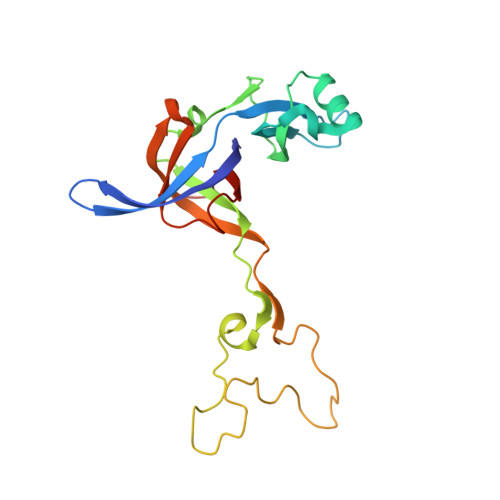
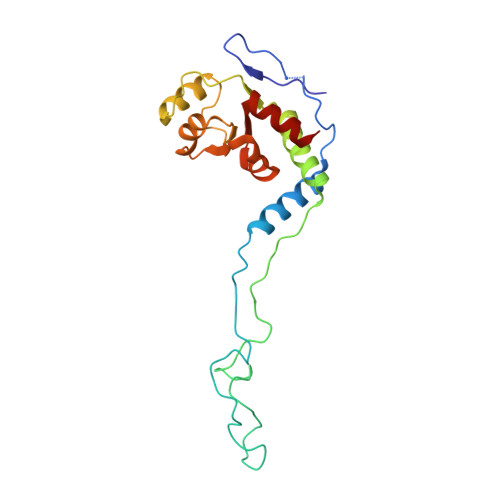
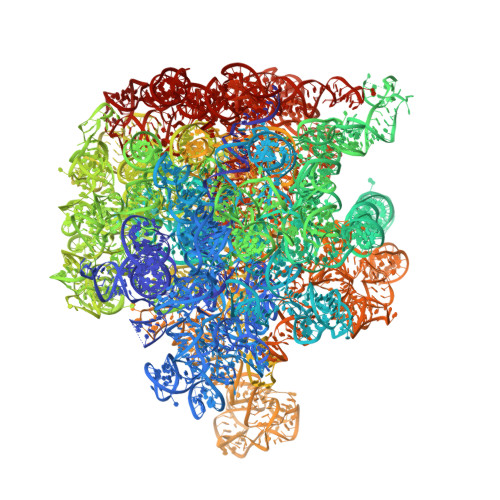
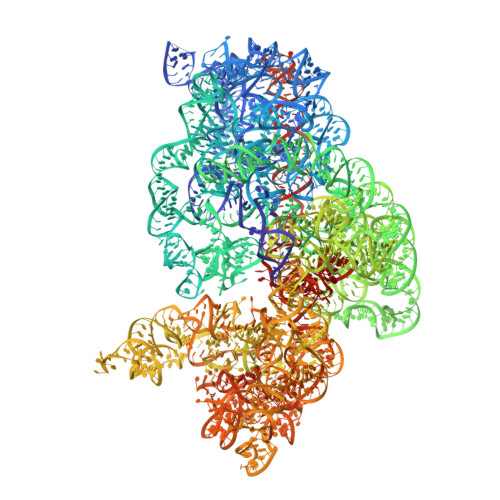
The antibiotic thermorubin inhibits protein synthesis by binding to inter-subunit bridge b2a of the ribosome.
Bulkley, D., Johnson, F., Steitz, T.A.(2012) J Mol Biol 416: 571-578
- PubMed: 22240456
- DOI: https://doi.org/10.1016/j.jmb.2011.12.055
- Primary Citation of Related Structures:
4V8A - PubMed Abstract:
Thermorubin is a small-molecule inhibitor of bacterial protein synthesis, but relatively little is known about the molecular mechanism by which it blocks translation. The structure of the complex between thermorubin and the 70S ribosome from Thermus thermophilus reported here shows that thermorubin interacts with the ribosome in a way that is distinct from any other known class of ribosome inhibitor. Though it is structurally similar to tetracycline, it binds to the ribosome at an entirely different location-the interface between the small and large subunits that is formed by inter-subunit bridge B2a. This region of the ribosome is known to play a role in the initiation of translation, and thus, the binding site we observe is consistent with evidence suggesting that thermorubin inhibits the initiation stage of protein synthesis. The binding of thermorubin induces a rearrangement of two bases on helix 69 of the 23S rRNA, and presumably, this rearrangement blocks the binding of an A-site tRNA, thereby inhibiting peptide bond formation. Due in part to its low solubility in aqueous media, thermorubin has not been used clinically, although it is a potent antibacterial agent with low toxicity (Therapeutic Index>200). The interactions between thermorubin and the ribosome, as well as its adjacency to the observed binding sites of three other antibiotic classes, may enable the design of novel derivatives that share thermorubin's mode of action but possess improved pharmacodynamic properties.
Organizational Affiliation:
Department of Chemistry, Yale University, New Haven, CT 06520-8107, USA.