Influence of weak-intensity data, ordered water molecules, and hydrogen atoms on the refinement of a large protein crystal structure
Wang, J., Lomkalin, I.V.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Catalase HPII | A [auth P], B [auth Q], C [auth R], D [auth S] | 753 | Escherichia coli | Mutation(s): 0 EC: 1.11.1.6 | 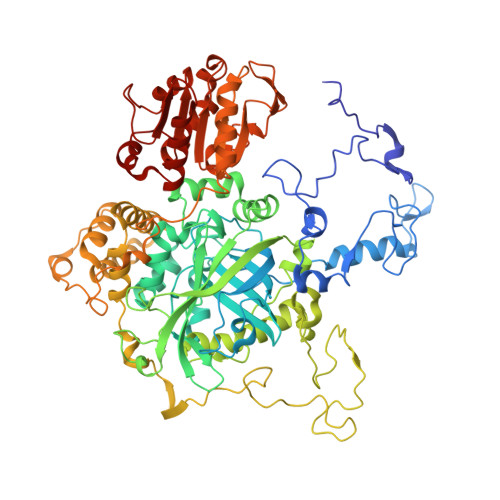 |
UniProt | |||||
Find proteins for P21179 (Escherichia coli (strain K12)) Explore P21179 Go to UniProtKB: P21179 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P21179 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 8 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HDD Query on HDD | E [auth P], HB [auth S], VA [auth R], W [auth Q] | CIS-HEME D HYDROXYCHLORIN GAMMA-SPIROLACTONE C34 H32 Fe N4 O5 UMGOPAWIGKFTRK-QQDQPIDJSA-N |  | ||
| PG4 Query on PG4 | GB [auth R] | TETRAETHYLENE GLYCOL C8 H18 O5 UWHCKJMYHZGTIT-UHFFFAOYSA-N |  | ||
| PGE Query on PGE | BB [auth R], CB [auth R], P | TRIETHYLENE GLYCOL C6 H14 O4 ZIBGPFATKBEMQZ-UHFFFAOYSA-N |  | ||
| PEG Query on PEG | R [auth P] RA [auth Q] S [auth P] SA [auth Q] T [auth P] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | UB [auth S], V [auth P] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | DB [auth R] EB [auth R] F [auth P] IB [auth S] PA [auth Q] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | AA [auth Q] AB [auth R] BA [auth Q] CA [auth Q] DA [auth Q] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| MG Query on MG | FB [auth R], VB [auth S] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 161.789 | α = 90 |
| b = 171.331 | β = 121.55 |
| c = 122.484 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-3000 | data reduction |
| SCALEPACK | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM022778 |