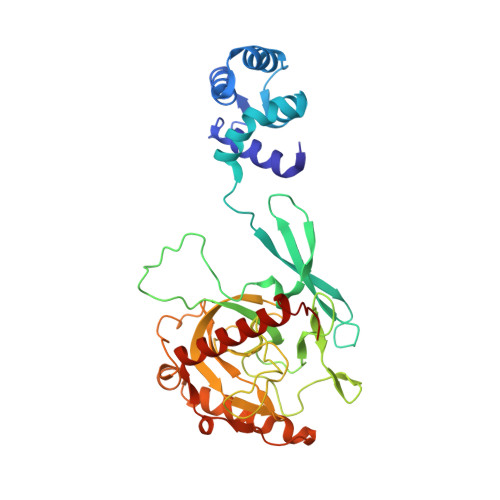
Structural and Functional Highlights of Vacuolar Soluble Protein 1 from Pathogen Trypanosoma brucei brucei
Jamwal, A., Round, A.R., Bannwarth, L., Venien-Bryan, C., Belrhali, H., Yogavel, M., Sharma, A.(2015) J Biological Chem 290: 30498-30513
- PubMed: 26494625
- DOI: https://doi.org/10.1074/jbc.M115.674176
- Primary Citation of Related Structures:
5C5V - PubMed Abstract:
Trypanosoma brucei (T. brucei) is responsible for the fatal human disease called African trypanosomiasis, or sleeping sickness. The causative parasite, Trypanosoma, encodes soluble versions of inorganic pyrophosphatases (PPase), also called vacuolar soluble proteins (VSPs), which are localized to its acidocalcisomes. The latter are acidic membrane-enclosed organelles rich in polyphosphate chains and divalent cations whose significance in these parasites remains unclear. We here report the crystal structure of T. brucei brucei acidocalcisomal PPases in a ternary complex with Mg(2+) and imidodiphosphate. The crystal structure reveals a novel structural architecture distinct from known class I PPases in its tetrameric oligomeric state in which a fused EF hand domain arranges around the catalytic PPase domain. This unprecedented assembly evident from TbbVSP1 crystal structure is further confirmed by SAXS and TEM data. SAXS data suggest structural flexibility in EF hand domains indicative of conformational plasticity within TbbVSP1.
- From the Structural and Computational Biology Group, International Centre for Genetic Engineering and Biotechnology, New Delhi 110067, India.
Organizational Affiliation: