Paying the Price of Desolvation in Solvent-Exposed Protein Pockets: Impact of Distal Solubilizing Groups on Affinity and Binding Thermodynamics in a Series of Thermolysin Inhibitors.
Cramer, J., Krimmer, S.G., Heine, A., Klebe, G.(2017) J Med Chem 60: 5791-5799
- PubMed: 28590130
- DOI: https://doi.org/10.1021/acs.jmedchem.7b00490
- Primary Citation of Related Structures:
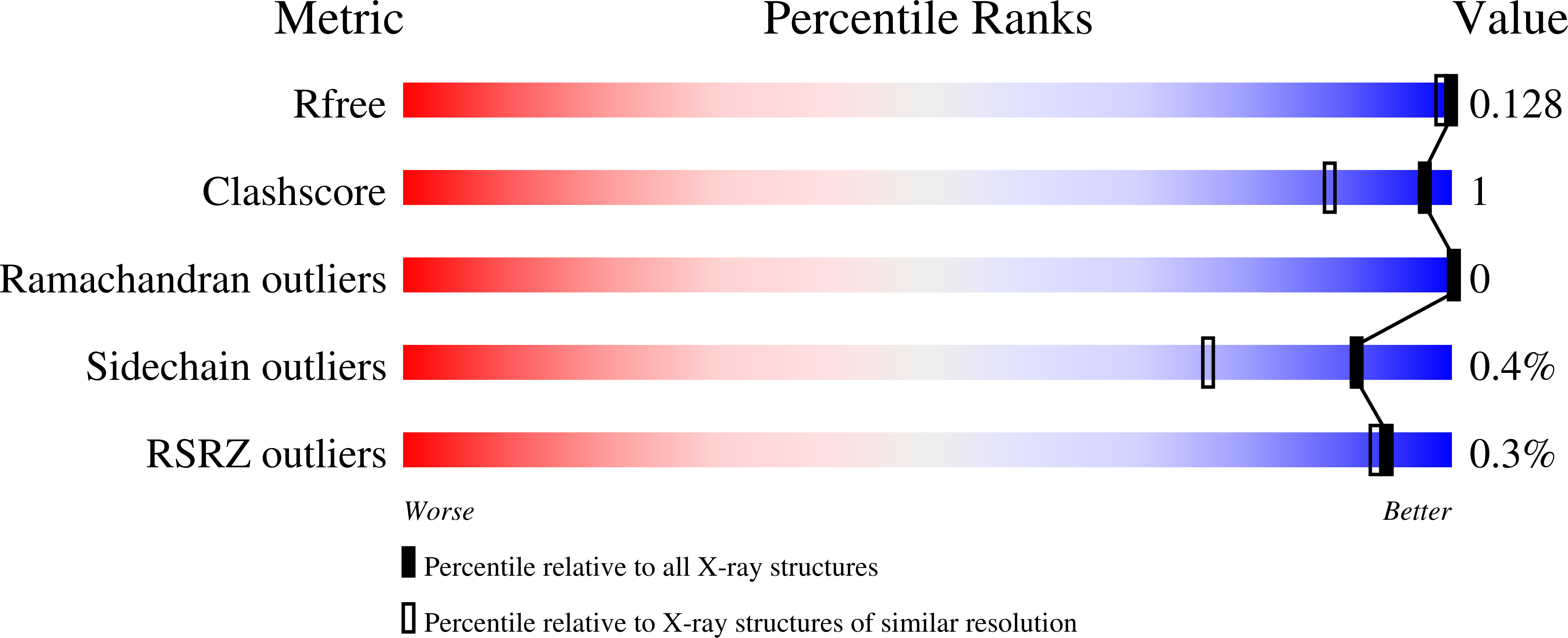
5MNR, 5N2T, 5N2X, 5N2Z, 5N31, 5N34, 5N3V, 5N3Y - PubMed Abstract:
In lead optimization, open, solvent-exposed protein pockets are often disregarded as prospective binding sites. Because of bulk-solvent proximity, researchers are instead enticed to attach charged polar groups at inhibitor scaffolds to improve solubility and pharmacokinetic properties. It is rarely considered that solvent effects from water reorganization in the first hydration shell of protein-ligand complexes can have a significant impact on binding. We investigate the thermodynamic fingerprint of thermolysin inhibitors featuring terminal charged ammonium groups that are gradually pulled from a distal, solvent-exposed position into the flat, bowl-shaped S 2 ' pocket. Even for the most remote attachment, costs for partial desolvation of the polar group next to the protein-solvent interface are difficult to compensate by interactions with the protein or surrounding water molecules. Through direct comparison with hydrophobic analogues, a significant 180-fold affinity loss was recorded, which questions popular strategies to attach polar ligand-solubilizing groups at the exposed terminus of substituents accommodated in flat open pockets.
Organizational Affiliation:
Institute of Pharmaceutical Chemistry, University of Marburg , Marbacher Weg 6, 35032 Marburg, Germany.