Discovery and optimization of 2-pyridinone aminal integrase strand transfer inhibitors for the treatment of HIV.
Schreier, J.D., Embrey, M.W., Raheem, I.T., Barbe, G., Campeau, L.C., Dubost, D., McCabe Dunn, J., Grobler, J., Hartingh, T.J., Hazuda, D.J., Klein, D., Miller, M.D., Moore, K.P., Nguyen, N., Pajkovic, N., Powell, D.A., Rada, V., Sanders, J.M., Sisko, J., Steele, T.G., Wai, J., Walji, A., Xu, M., Coleman, P.J.(2017) Bioorg Med Chem Lett 27: 2038-2046
- PubMed: 28285916
- DOI: https://doi.org/10.1016/j.bmcl.2017.02.039
- Primary Citation of Related Structures:
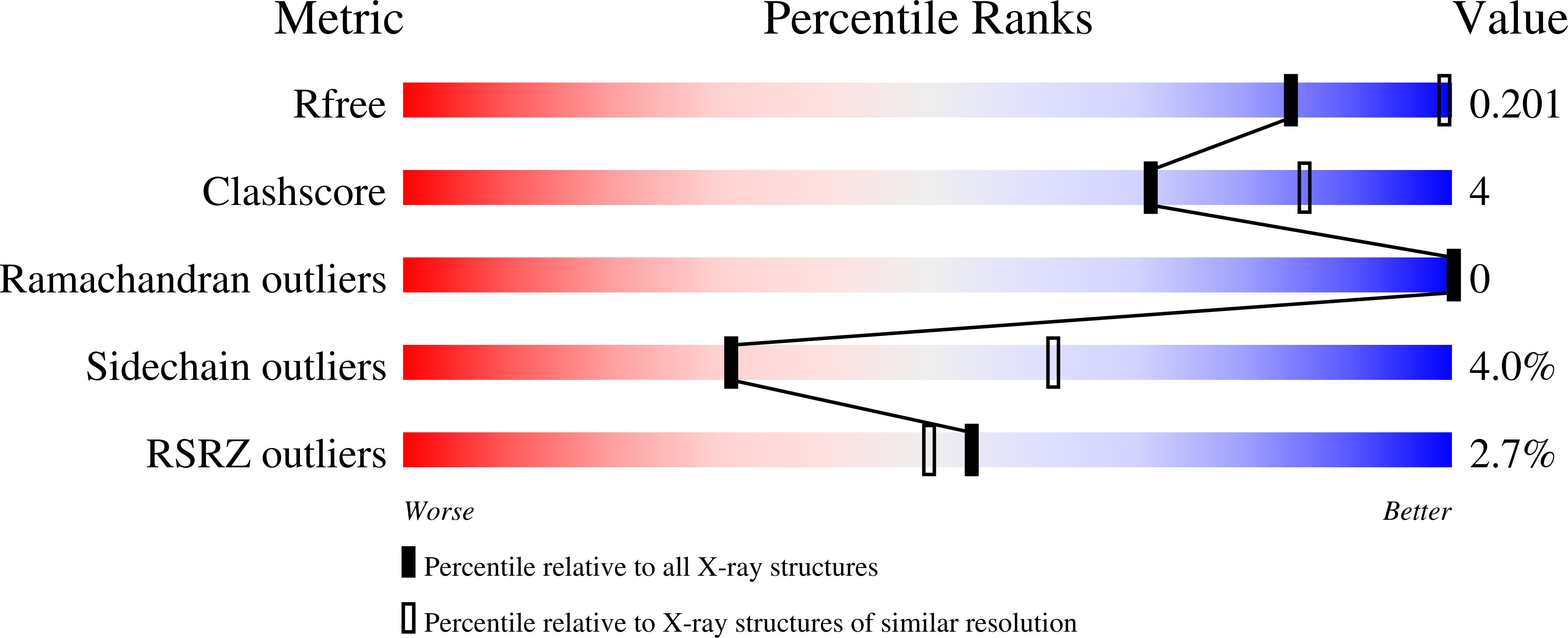
5UOP, 5UOQ - PubMed Abstract:
HIV integrase strand transfer inhibitors (InSTIs) represent an important class of antiviral therapeutics with proven efficacy and excellent tolerability for the treatment of HIV infections. In 2007, Raltegravir became the first marketed strand transfer inhibitor pioneering the way to a first-line therapy for treatment-naïve patients. Challenges with this class of therapeutics remain, including frequency of the dosing regimen and the genetic barrier to resistance. To address these issues, research towards next-generation integrase inhibitors has focused on imparting potency against RAL-resistent mutants and improving pharmacokinetic profiles. Herein, we detail medicinal chemistry efforts on a novel class of 2-pyridinone aminal InSTIs, inpsired by MK-0536, which led to the discovery of important lead molecules for our program. Systematic optimization carried out at the amide and aminal positions on the periphery of the core provided the necessary balance of antiviral activity and physiochemical properties. These efforts led to a novel aminal lead compound with the desired virological profile and preclinical pharmacokinetic profile to support a once-daily human dose prediction.
Organizational Affiliation:
Discovery Chemistry, Merck Research Laboratories, West Point, PA 19486, United States. Electronic address: john_schreier@merck.com.