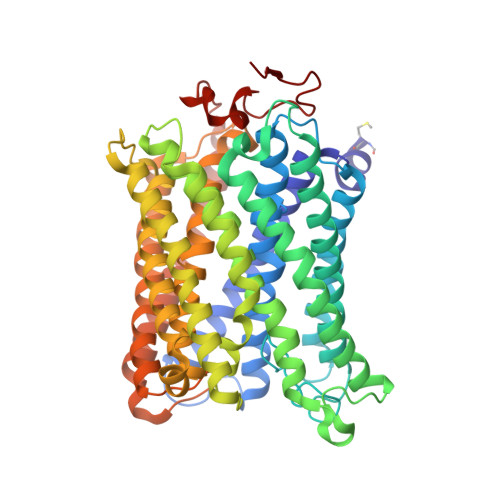
Structure of bovine cytochrome c oxidase crystallized at a neutral pH using a fluorinated detergent.
Luo, F., Shinzawa-Itoh, K., Hagimoto, K., Shimada, A., Shimada, S., Yamashita, E., Yoshikawa, S., Tsukihara, T.(2017) Acta Crystallogr F Struct Biol Commun 73: 416-422
- PubMed: 28695851
- DOI: https://doi.org/10.1107/S2053230X17008834
- Primary Citation of Related Structures:
5XDQ - PubMed Abstract:
Cytochrome c oxidase (CcO) couples proton pumping to O 2 reduction. Its enzymatic activity depends sensitively on pH over a wide range. However, owing to difficulty in crystallizing this protein, X-ray structure analyses of bovine CcO aimed at understanding its reaction mechanism have been conducted using crystals prepared at pH 5.7, which is significantly lower than that in the cell. Here, oxidized CcO at pH 7.3 was crystallized using a fluorinated octyl-maltoside derivative, and the structure was determined at 1.77 Å resolution. No structural differences between crystals obtained at the neutral pH and the acidic pH were detected within the molecules. On the other hand, some differences in intermolecular interactions were detected between the two types of crystal. The influence of pH on the molecular surface is likely to contribute to the pH dependency of the aerobic oxidation of ferrocytochrome c.
- Picobiology Institute, Graduate School of Life Science, University of Hyogo, 3-2-1 Koto, Kamigori-cho, Ako-gun, Hyogo 678-1297, Japan.
Organizational Affiliation: