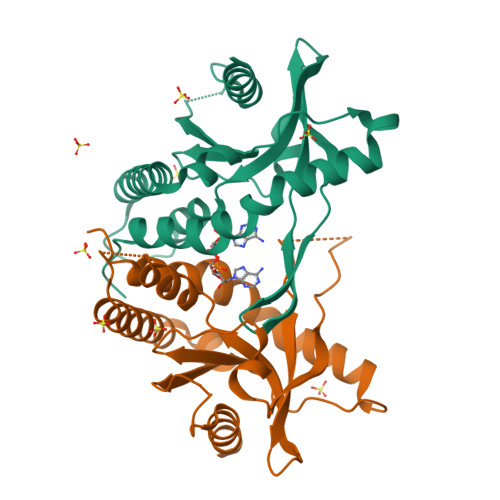
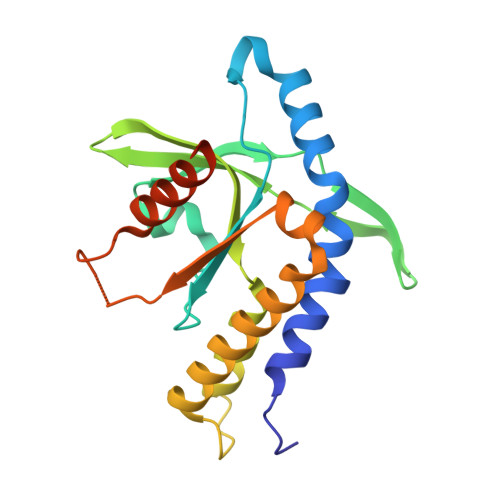
Crystal structures of porcine STINGCBD-CDN complexes reveal the mechanism of ligand recognition and discrimination of STING proteins.
Cong, X., Yuan, Z., Du, Y., Wu, B., Lu, D., Wu, X., Zhang, Y., Li, F., Wei, B., Li, J., Wu, J., Xu, S., Wang, J., Qi, J., Shang, G., Gu, L.(2019) J Biological Chem 294: 11420-11432
- PubMed: 31167783
- DOI: https://doi.org/10.1074/jbc.RA119.007367
- Primary Citation of Related Structures:
6A03, 6A04, 6A05, 6A06, 6IYF - PubMed Abstract:
The cyclic dinucleotide (CDN)- st imulator of in terferon g enes (STING) pathway plays an important role in the detection of viral and bacterial pathogens in animals. Previous studies have shown that the metazoan second messenger cyclic [G(2',5')pA(3',5')p] (2',3'-cGAMP) generated by cyclic GMP-AMP synthase cGAS binds STING with high affinity compared with bacterial CDNs such as c-di-GMP, c-di-AMP, and 3',3'-cGAMP. Despite recent progress indicating that the CDN-binding domain (CBD) of dimeric STING binds asymmetric 2',3'-cGAMP preferentially over symmetric 3',3'-CDNs, it remains an open question whether STING molecules, such as human STING, adopt a symmetric dimeric conformation to efficiently engage its asymmetric ligand. Here, structural studies of the CBD from porcine STING (STING CBD ) in complex with CDNs at 1.76-2.6 Å resolution revealed that porcine STING CBD , unlike its human and mouse counterparts, can adopt an asymmetric ligand-binding pocket to accommodate the CDNs. We observed that the extensive interactions and shape complementarity between asymmetric 2',3'-cGAMP and the ligand-binding pocket make it the most preferred ligand for porcine STING and that geometry constraints limit the binding between symmetric 3',3'-CDN and porcine STING. The ligand-discrimination mechanism of porcine STING observed here expands our understanding of how the CDN-STING pathway is activated and of its role in antiviral defense.
Organizational Affiliation:
State Key Laboratory of Microbial Technology, Shandong University, Jinan 250100, China.