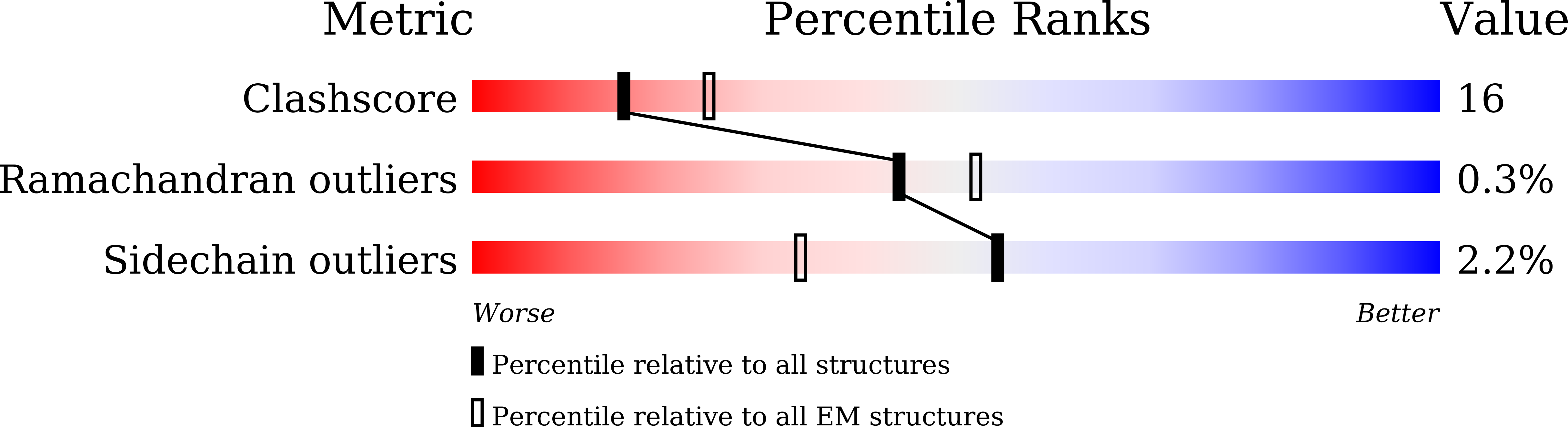Neutralization sites of human papillomavirus-6 relate to virus attachment and entry phase in viral infection.
Liu, X., Chen, J., Wang, Z., Wang, D., He, M., Qian, C., Song, S., Chi, X., Kong, Z., Zheng, Q., Wang, Y., Yu, H., Zhao, Q., Zhang, J., Li, S., Gu, Y., Xia, N.(2019) Emerg Microbes Infect 8: 1721-1733
- PubMed: 31769733
- DOI: https://doi.org/10.1080/22221751.2019.1694396
- Primary Citation of Related Structures:
6L31 - PubMed Abstract:
Human papillomavirus type 6 (HPV6) is the major etiologic agent of genital warts and recurrent respiratory papillomatosis. Although the commercial HPV vaccines cover HPV6, the neutralization sites and mode for HPV6 are poorly understood. Here, we identify the HPV6 neutralization sites and discriminate the inhibition of virus attachment and entry by three potent neutralizing antibodies (nAbs), 5D3, 17D5, and 15F7. Mutagenesis assays showed that these nAbs predominantly target surface loops BC, DE, and FG of HPV6 L1. Cryo-EM structures of the HPV6 pseudovirus (PsV) and its immune complexes revealed three distinct binding modalities - full-occupation-bound to capsid, top-center-bound-, and top-rim-bound to pentamers - and illustrated a structural atlas for three classes of antibody-bound footprints that are located at center-distal ring, center, and center-proximal ring of pentamer surface for 5D3, 17D5, and 15F7, respectively. Two modes of neutralization were identified: mAb 5D3 and 17D5 block HPV PsV from attaching to the extracellular matrix (ECM) and the cell surface, whereas 15F7 allows PsV attachment but prohibits PsV from entering the cell. These findings highlight three neutralization sites of HPV6 L1 and outline two antibody-mediated neutralization mechanisms against HPV6, which will be relevant for HPV virology and antiviral inhibitor design. HighlightsMajor neutralization sites of HPV6 were mapped on the pseudovirus cryo-EM structuremAb 15F7 binds HPV6 capsid with a novel top-rim binding modality and confers a post-attachment neutralizationmAb 17D5 binds capsid in top-centre manner but unexpectedly prevents virus from attachment to cell surface.
Organizational Affiliation:
State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Life Sciences, Xiamen University, Xiamen, People's Republic of China.