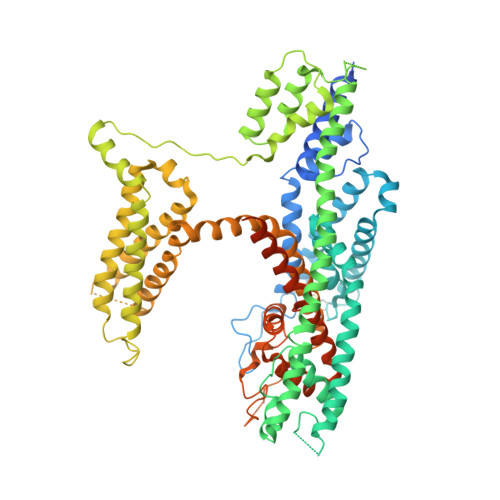
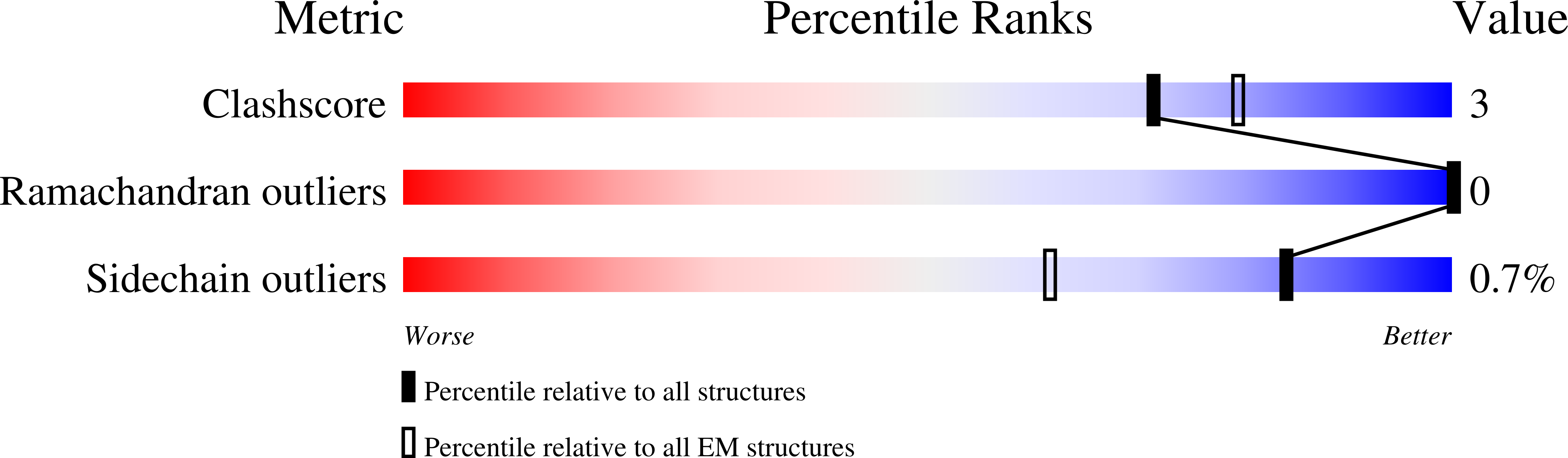Structural mechanisms of phospholipid activation of the human TPC2 channel.
She, J., Zeng, W., Guo, J., Chen, Q., Bai, X., Jiang, Y.(2019) Elife 8
- PubMed: 30860481
- DOI: https://doi.org/10.7554/eLife.45222
- Primary Citation of Related Structures:
6NQ0, 6NQ1, 6NQ2 - PubMed Abstract:
Mammalian two-pore channels (TPCs) regulate the physiological functions of the endolysosome. Here we present cryo-EM structures of human TPC2 (HsTPC2), a phosphatidylinositol 3,5-bisphosphate (PI(3,5)P 2 )-activated, Na + selective channel, in the ligand-bound and apo states. The apo structure captures the closed conformation, while the ligand-bound form features the channel in both open and closed conformations. Combined with functional analysis, these structures provide insights into the mechanism of PI(3,5)P 2 -regulated gating of TPC2, which is distinct from that of TPC1. Specifically, the endolysosome-specific PI(3,5)P 2 binds at the first 6-TM and activates the channel - independently of the membrane potential - by inducing a structural change at the pore-lining inner helix (IS6), which forms a continuous helix in the open state but breaks into two segments at Gly317 in the closed state. Additionally, structural comparison to the voltage-dependent TPC1 structure allowed us to identify Ile551 as being responsible for the loss of voltage dependence in TPC2.
Organizational Affiliation:
Department of Physiology, University of Texas Southwestern Medical Center, Dallas, United States.