Cellular and biochemical validation of a potent PAICS inhibitor
Marttila, P., Skerlova, J., Unterlass, J., Jemth, A.-S., Henriksson, M., Wakchaure, P., Grube, M., Warpman Berglund, U., Homan, E., Stenmark, P., Helleday, T.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Multifunctional protein ADE2 | 425 | Homo sapiens | Mutation(s): 0 Gene Names: PAICS, ADE2, AIRC, PAIS EC: 6.3.2.6 (PDB Primary Data), 4.1.1.21 (PDB Primary Data) | 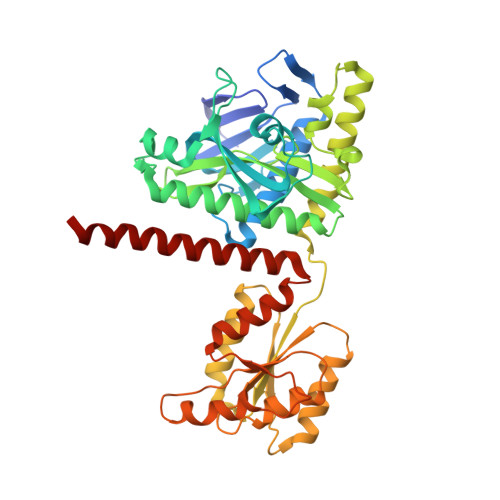 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P22234 (Homo sapiens) Explore P22234 Go to UniProtKB: P22234 | |||||
PHAROS: P22234 GTEx: ENSG00000128050 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P22234 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| RLK (Subject of Investigation/LOI) Query on RLK | C [auth A], E [auth B] | 2-azanyl-~{N}-[2-bromanyl-5-[4-[3-(dimethylamino)propylsulfonyl]piperazin-1-yl]phenyl]-1,3-oxazole-4-carboxamide C19 H27 Br N6 O4 S GTJMWLKWMPRNKA-UHFFFAOYSA-N |  | ||
| OK8 (Subject of Investigation/LOI) Query on OK8 | D [auth A], F [auth B] | (2~{S})-2-[[5-azanyl-1-[(2~{R},3~{R},4~{S},5~{R})-3,4-bis(oxidanyl)-5-(phosphonooxymethyl)oxolan-2-yl]imidazol-4-yl]car
bonylamino]butanedioic acid C13 H19 N4 O12 P NAQGHJTUZRHGAC-ZZZDFHIKSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 60.772 | α = 90 |
| b = 152.44 | β = 90 |
| c = 222.755 | γ = 90 |
| Software Name | Purpose |
|---|---|
| XSCALE | data scaling |
| MOLREP | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Swedish Research Council | Sweden | 2015-00162 |
| Swedish Research Council | Sweden | 2018-03406 |
| European Research Council (ERC) | European Union | TAROX-695376 |
| European Regional Development Fund | European Union | CZ.02.2.69/0.0/0.0/16_027/0008477 |