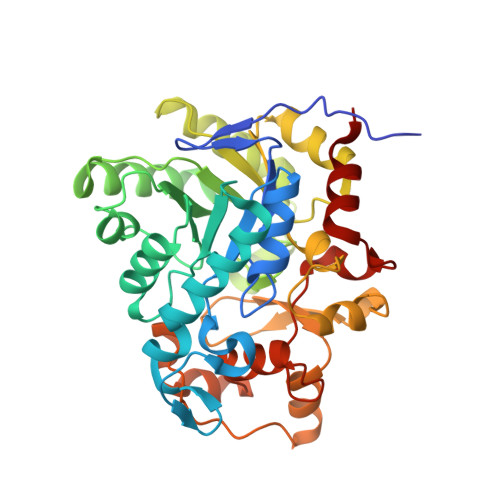
Helicobacter pylori FabX contains a [4Fe-4S] cluster essential for unsaturated fatty acid synthesis.
Zhou, J., Zhang, L., Zeng, L., Yu, L., Duan, Y., Shen, S., Hu, J., Zhang, P., Song, W., Ruan, X., Jiang, J., Zhang, Y., Zhou, L., Jia, J., Hang, X., Tian, C., Lin, H., Chen, H.Z., Cronan, J.E., Bi, H., Zhang, L.(2021) Nat Commun 12: 6932-6932
- PubMed: 34836944
- DOI: https://doi.org/10.1038/s41467-021-27148-0
- Primary Citation of Related Structures:
7E1Q, 7E1R, 7E1S - PubMed Abstract:
Unsaturated fatty acids (UFAs) are essential for functional membrane phospholipids in most bacteria. The bifunctional dehydrogenase/isomerase FabX is an essential UFA biosynthesis enzyme in the widespread human pathogen Helicobacter pylori, a bacterium etiologically related to 95% of gastric cancers. Here, we present the crystal structures of FabX alone and in complexes with an octanoyl-acyl carrier protein (ACP) substrate or with holo-ACP. FabX belongs to the nitronate monooxygenase (NMO) flavoprotein family but contains an atypical [4Fe-4S] cluster absent in all other family members characterized to date. FabX binds ACP via its positively charged α7 helix that interacts with the negatively charged α2 and α3 helices of ACP. We demonstrate that the [4Fe-4S] cluster potentiates FMN oxidation during dehydrogenase catalysis, generating superoxide from an oxygen molecule that is locked in an oxyanion hole between the FMN and the active site residue His182. Both the [4Fe-4S] and FMN cofactors are essential for UFA synthesis, and the superoxide is subsequently excreted by H. pylori as a major resource of peroxide which may contribute to its pathogenic function in the corrosion of gastric mucosa.
Organizational Affiliation:
Department of Pharmacology and Chemical Biology, State Key Laboratory of Oncogenes and Related Genes, Shanghai Jiao Tong University School of Medicine, 200025, Shanghai, China.