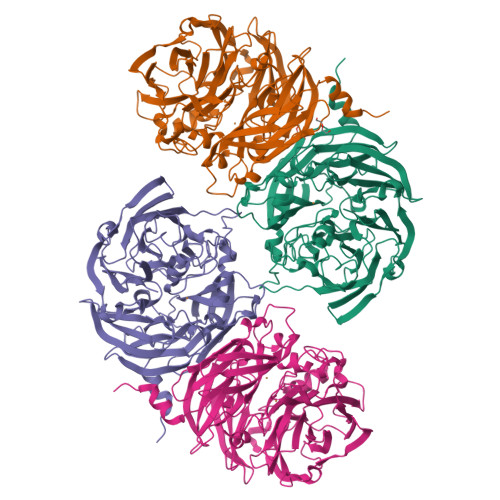
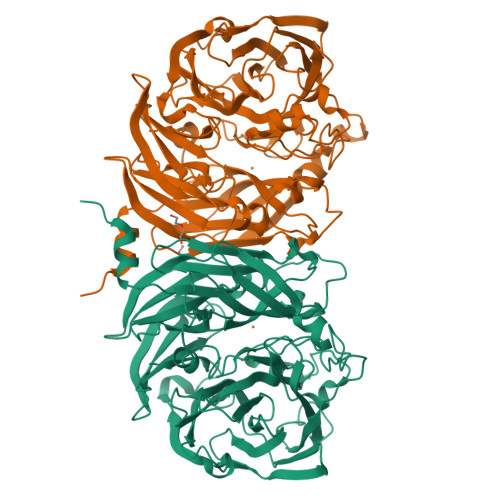
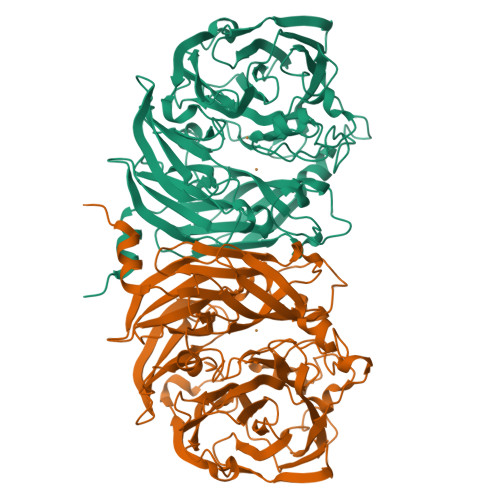
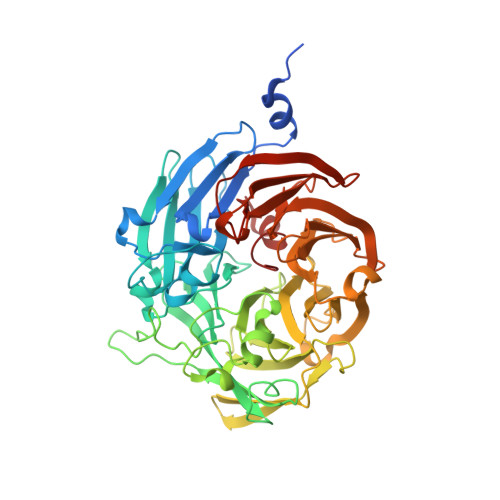
Probing the Role of a Conserved Phenylalanine in the Active Site of Thiocyanate Dehydrogenase
Varfolomeeva, L.A., Solovieva, A.Y., Shipkov, N.S., Kulikova, O.G., Dergousova, N.I., Rakitina, T.V., Boyko, K.M., Tikhonova, T.V., Popov, V.O.(2022) Crystals (Basel) 12