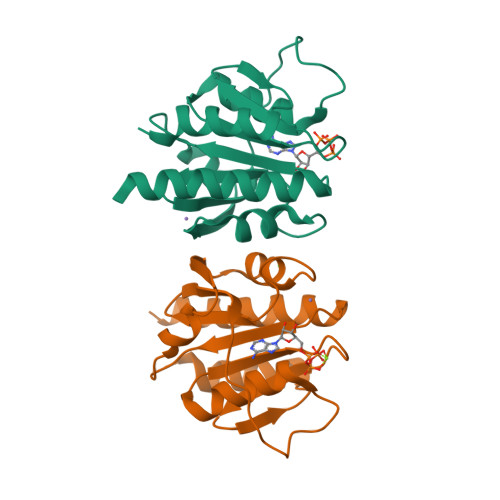
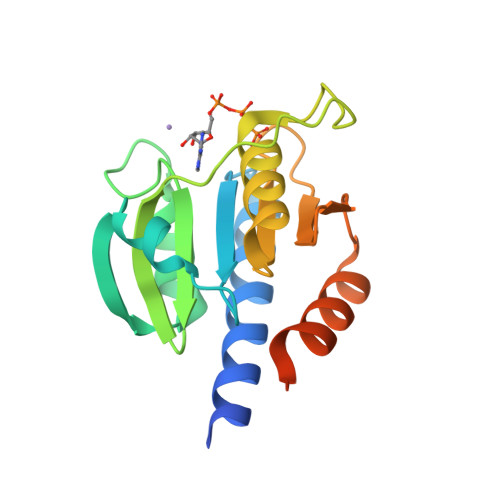
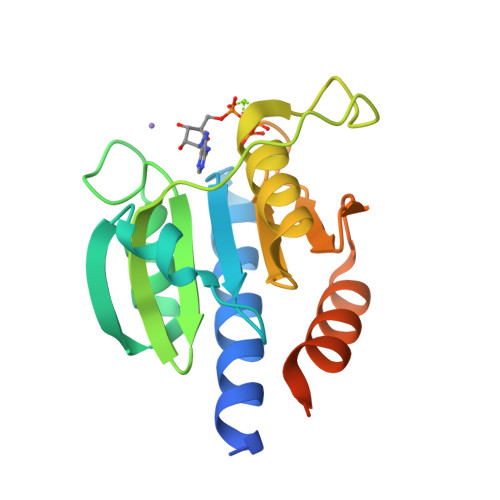
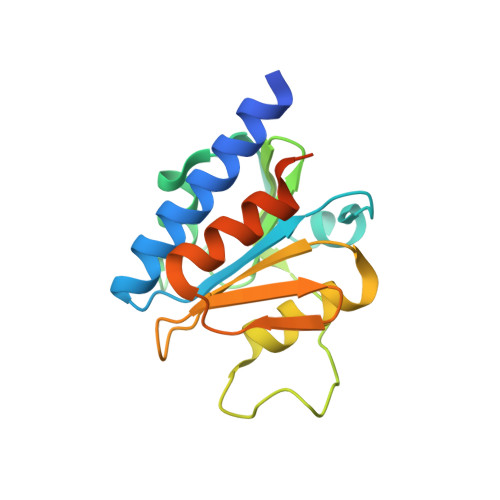
Computer-aided design of a cyclic di-AMP synthesizing enzyme CdaA inhibitor.
Neumann, P., Kloskowski, P., Ficner, R.(2023) Microlife 4: uqad021-uqad021
- PubMed: 37223749
- DOI: https://doi.org/10.1093/femsml/uqad021
- Primary Citation of Related Structures:
8C4J, 8C4M, 8C4N, 8C4O, 8C4P, 8C4Q, 8C4R - PubMed Abstract:
Cyclic di-AMP (c-di-AMP) is an essential secondary messenger regulating cell wall homeostasis and myriads of physiological processes in several Gram-positive and mycobacteria, including human pathogens. Hence, c-di-AMP synthesizing enzymes (DACs) have become a promising antibacterial drug target. To overcome a scarcity of small molecule inhibitors of c-di-AMP synthesizing enzyme CdaA, a computer-aided design of a new compound that should block the enzyme has been performed. This has led to the identification of a molecule comprising two thiazole rings and showing inhibitory potential based on ITC measurements. Thiazole scaffold is a good pharmacophore nucleus known due to its various pharmaceutical applications. It is contained in more than 18 FDA-approved drugs as well as in dozens of experimental drugs. Hence, the designed inhibitor can serve as a potent lead compound for further development of inhibitor against CdaA.
Organizational Affiliation:
Department of Molecular Structural Biology, Georg-August-University Goettingen, Institute of Microbiology and Genetics, GZMB, Justus-von-Liebig Weg 11, 37077 Goettingen, Germany.