Mutational fitness landscape of human influenza H3N2 neuraminidase.
Lei, R., Hernandez Garcia, A., Tan, T.J.C., Teo, Q.W., Wang, Y., Zhang, X., Luo, S., Nair, S.K., Peng, J., Wu, N.C.(2023) Cell Rep 42: 111951-111951
- PubMed: 36640354
- DOI: https://doi.org/10.1016/j.celrep.2022.111951
- Primary Citation of Related Structures:
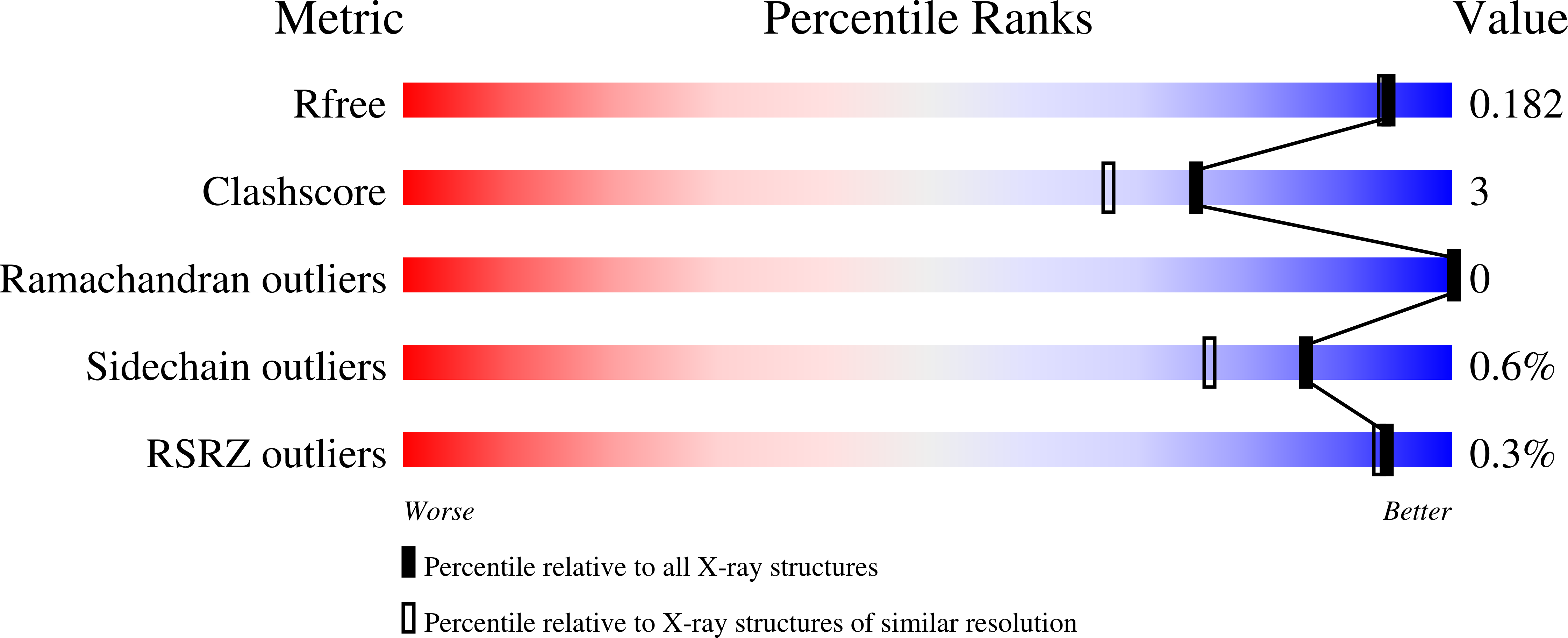
8DWB - PubMed Abstract:
Influenza neuraminidase (NA) has received increasing attention as an effective vaccine target. However, its mutational tolerance is not well characterized. Here, the fitness effects of >6,000 mutations in human H3N2 NA are probed using deep mutational scanning. Our result shows that while its antigenic regions have high mutational tolerance, there are solvent-exposed regions with low mutational tolerance. We also find that protein stability is a major determinant of NA mutational fitness. The deep mutational scanning result correlates well with mutational fitness inferred from natural sequences using a protein language model, substantiating the relevance of our findings to the natural evolution of circulating strains. Additional analysis further suggests that human H3N2 NA is far from running out of mutations despite already evolving for >50 years. Overall, this study advances our understanding of the evolutionary potential of NA and the underlying biophysical constraints, which in turn provide insights into NA-based vaccine design.
Organizational Affiliation:
Department of Biochemistry, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.