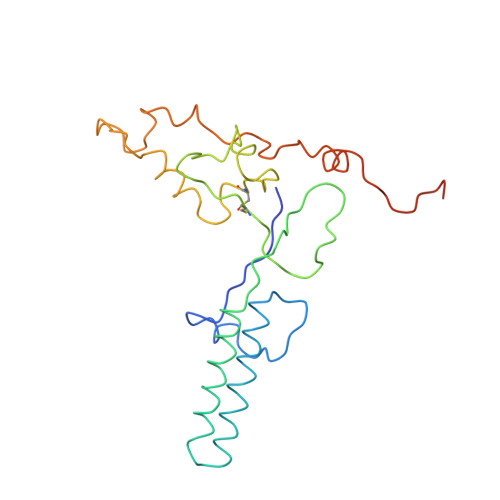
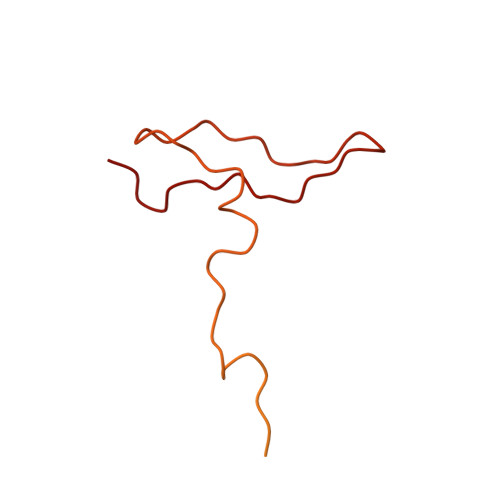
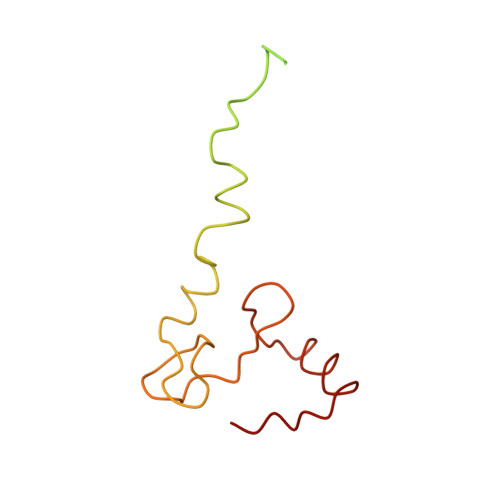
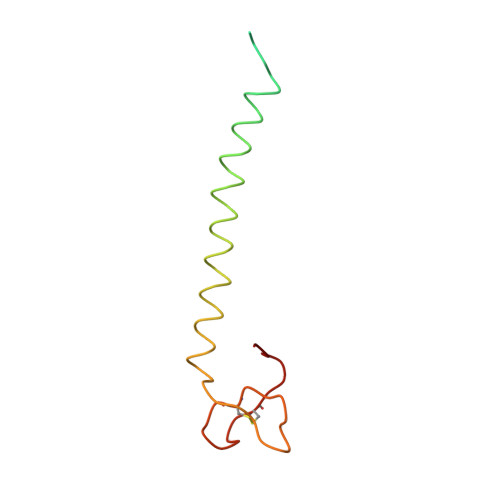
Near-atomic, non-icosahedrally averaged structure of giant virus Paramecium bursaria chlorella virus 1.
Shao, Q., Agarkova, I.V., Noel, E.A., Dunigan, D.D., Liu, Y., Wang, A., Guo, M., Xie, L., Zhao, X., Rossmann, M.G., Van Etten, J.L., Klose, T., Fang, Q.(2022) Nat Commun 13: 6476-6476
- PubMed: 36309542
- DOI: https://doi.org/10.1038/s41467-022-34218-4
- Primary Citation of Related Structures:
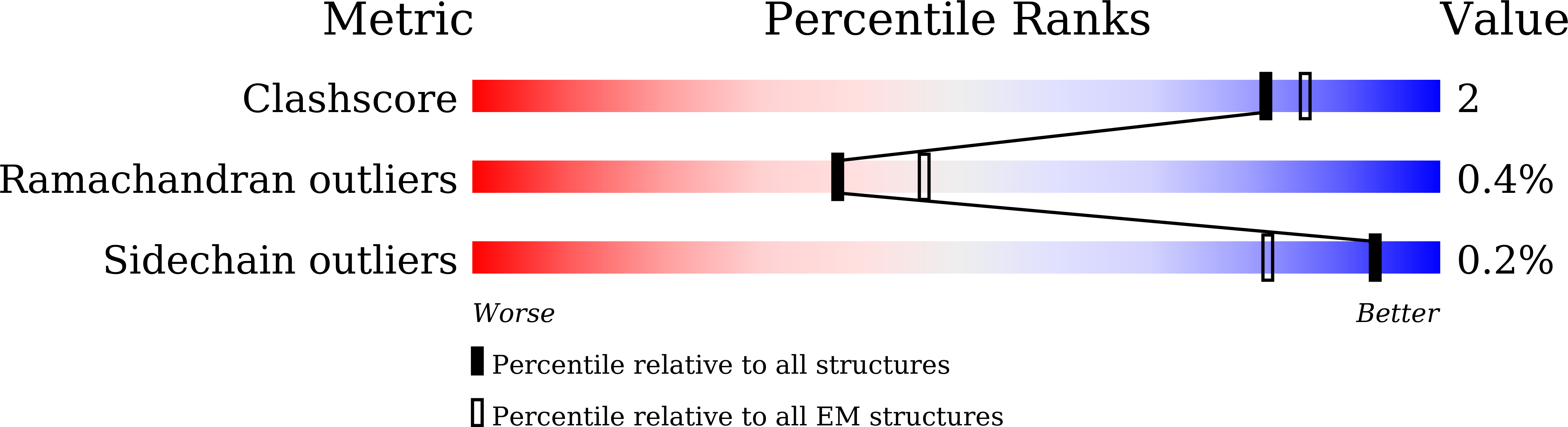
8H2I - PubMed Abstract:
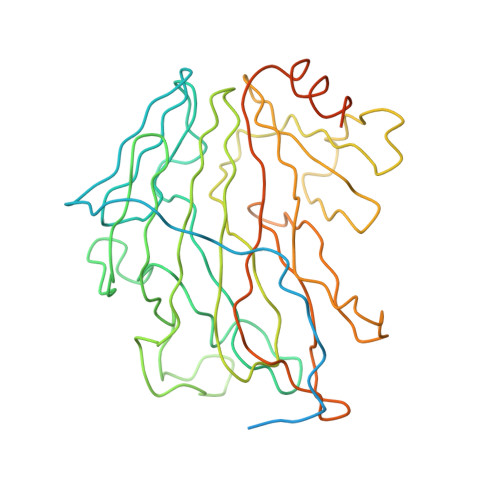
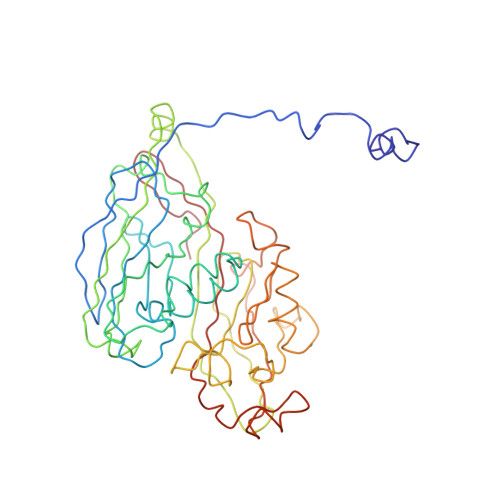
Giant viruses are a large group of viruses that infect many eukaryotes. Although components that do not obey the overall icosahedral symmetry of their capsids have been observed and found to play critical roles in the viral life cycles, identities and high-resolution structures of these components remain unknown. Here, by determining a near-atomic-resolution, five-fold averaged structure of Paramecium bursaria chlorella virus 1, we unexpectedly found the viral capsid possesses up to five major capsid protein variants and a penton protein variant. These variants create varied capsid microenvironments for the associations of fibers, a vesicle, and previously unresolved minor capsid proteins. Our structure reveals the identities and atomic models of the capsid components that do not obey the overall icosahedral symmetry and leads to a model for how these components are assembled and initiate capsid assembly, and this model might be applicable to many other giant viruses.
- Scholl of Public Health (Shenzhen), Sun Yat-sen University, Shenzhen, Guangdong, 518107, China.
Organizational Affiliation: