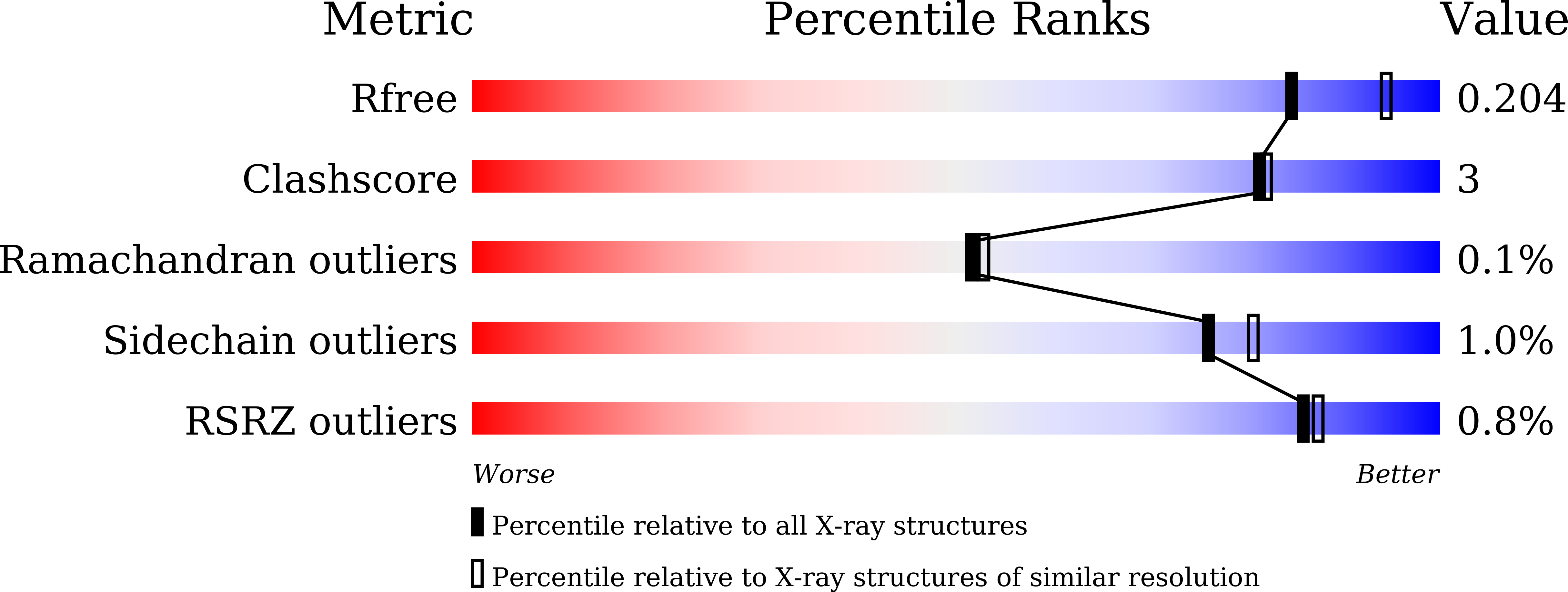
Revealing the intricate mechanism governing the pH-dependent activity of a quintessential representative of flavoproteins, glucose oxidase
Tu, T., Zhang, Y., Yan, Y., Li, L., Liu, X., Hakulinen, N., Zhang, W., Mu, Y., Luo, H., Yao, B., Li, W., Huang, H.(2024) Fundam Res