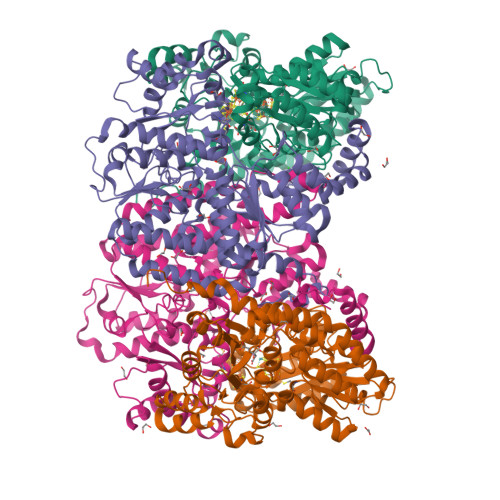
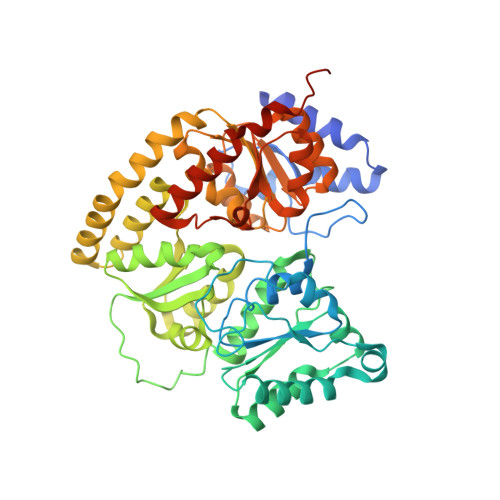
The Mononuclear Metal-Binding Site of Mo-Nitrogenase Is Not Required for Activity.
Cadoux, C., Maslac, N., Di Luzio, L., Ratcliff, D., Gu, W., Wagner, T., Milton, R.D.(2023) JACS Au 3: 2993-2999
- PubMed: 38034976
- DOI: https://doi.org/10.1021/jacsau.3c00567
- Primary Citation of Related Structures:
8P8G - PubMed Abstract:
The biological N 2 -fixation process is catalyzed exclusively by metallocofactor-containing nitrogenases. Structural and spectroscopic studies highlighted the presence of an additional mononuclear metal-binding (MMB) site, which can coordinate Fe in addition to the two metallocofactors required for the reaction. This MMB site is located 15-Å from the active site, at the interface of two NifK subunits. The enigmatic function of the MMB site and its implications for metallocofactor installation, catalysis, electron transfer, or structural stability are investigated in this work. The axial ligands coordinating the additional Fe are almost universally conserved in Mo-nitrogenases, but a detailed observation of the available structures indicates a variation in occupancy or a metal substitution. A nitrogenase variant in which the MMB is disrupted was generated and characterized by X-ray crystallography, biochemistry, and enzymology. The crystal structure refined to 1.55-Å revealed an unambiguous loss of the metal site, also confirmed by an absence of anomalous signal for Fe. The position of the surrounding side chains and the overall architecture are superposable with the wild-type structure. Accordingly, the biochemical and enzymatic properties of the variant are similar to those of the wild-type nitrogenase, indicating that the MMB does not impact nitrogenase's activity and stability in vitro .
Organizational Affiliation:
Department of Inorganic and Analytical Chemistry, Faculty of Sciences, University of Geneva, Quai Ernest-Ansermet 30, 1211 Geneva 4, Switzerland.