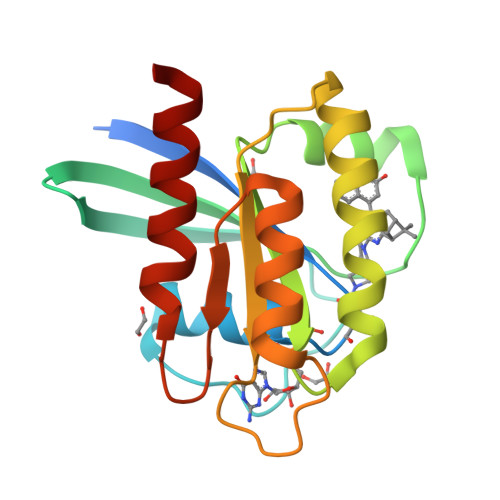
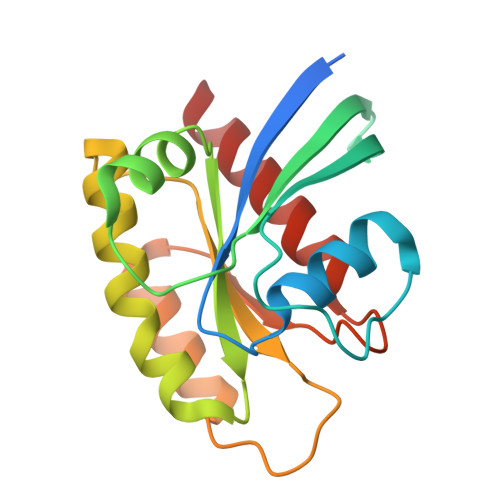
Identification of Structurally Novel KRAS G12C Inhibitors through Covalent DNA-Encoded Library Screening.
Huang, D., Manoni, F., Sun, Z., Liu, R., Allen, J.R., Banerjee, A., Cee, V.J., Eshon, J., Frohn, M.J., Kaller, M.R., Lee, H., Li, C., Li, X., Lopez, P., Ma, V., Medina, J.M., Mohr, C., Mukhina, O.A., Pickrell, A.J., Stellwagen, J., Wu, W., Zhang, W., Zhu, K., Dahal, U.P., Hu, L.A., Leavitt, M., Li, W., Li, Y., Ma, Y., Rex, K., Saiki, A.Y., Wang, P., Sun, Y., Dai, D., Tamayo, N.A., Lanman, B.A.(2025) J Med Chem 68: 4801-4817
- PubMed: 39930787
- DOI: https://doi.org/10.1021/acs.jmedchem.4c03071
- Primary Citation of Related Structures:
9E9H, 9E9I - PubMed Abstract:
Covalent inhibition of the KRAS G12C oncoprotein has emerged as a promising therapeutic approach for the treatment of nonsmall cell lung cancer (NSCLC). The identification of KRAS G12C inhibitors has typically relied on the high-throughput screening (HTS) of libraries of cysteine-reactive small molecules or on the attachment of cysteine-reactive warheads to noncovalent binders of KRAS. Such screening approaches have historically been limited in the size and diversity of molecules that could be effectively screened. DNA-encoded library (DEL) screening has emerged as a promising approach to accelerate the preparation and screening of incredibly large and diverse chemical libraries. Here, we describe the design and synthesis of a covalent DEL to screen ∼16 million compounds against KRAS G12C . We additionally describe the hit identification, validation, and structure-based optimization that culminated in the identification of a series of structurally novel, potent, and selective covalent inhibitors of KRAS G12C with good pharmacokinetic profiles and promising in vivo pharmacodynamic effects.
Organizational Affiliation:
Department of Therapeutic Discovery, Amgen Asia R&D Center, Amgen Research, 4560 Jinke Road, Pudong, Shanghai 201210, P. R. China.