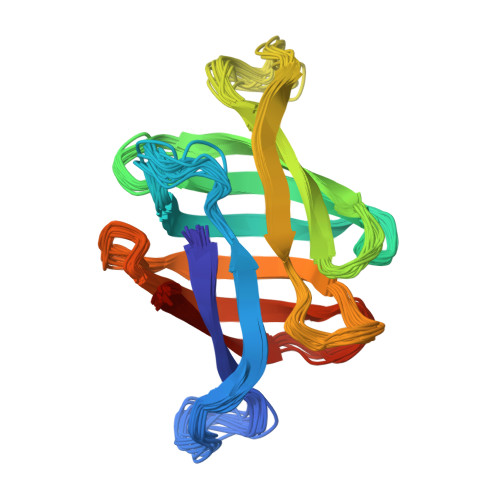
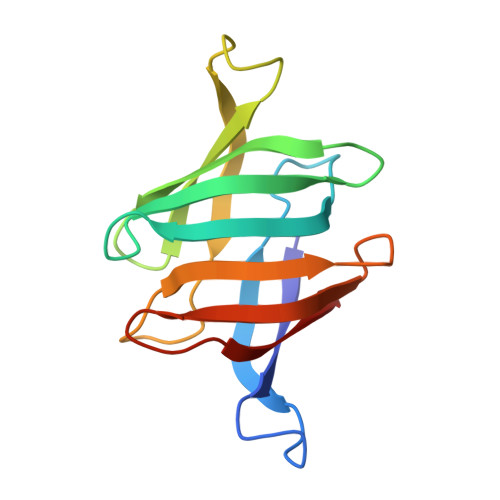
Sampling of Glycan-Bound Conformers by the Anti-HIV Lectin Oscillatoria agardhii agglutinin in the Absence of Sugar.
Carneiro, M.G., Koharudin, L.M., Ban, D., Sabo, T.M., Trigo-Mourino, P., Mazur, A., Griesinger, C., Gronenborn, A.M., Lee, D.(2015) Angew Chem Int Ed Engl 54: 6462-6465
- PubMed: 25873445
- DOI: https://doi.org/10.1002/anie.201500213
- Primary Citation of Related Structures:
2MWH - PubMed Abstract:
Lectins from different sources have been shown to interfere with HIV infection by binding to the sugars of viral-envelope glycoproteins. Three-dimensional atomic structures of a number of HIV-inactivating lectins have been determined, both as free proteins and in glycan-bound forms. However, details on the mechanism of recognition and binding to sugars are elusive. Herein we focus on the anti-HIV lectin OAA from Oscillatoria agardhii: We show that in the absence of sugars in solution, both the sugar-free and sugar-bound protein conformations that were observed in the X-ray crystal structures exist as conformational substates. Our results suggest that glycan recognition occurs by conformational selection within the ground state; this model differs from the popular "excited-state" model. Our findings provide further insight into molecular recognition of the major receptor on the HIV virus by OAA. These details can potentially be used for the optimization and/or development of preventive anti-HIV therapeutics.
Organizational Affiliation:
Department for NMR-Based Structural Biology, Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, 37077 Göttingen (Germany).