Unique binding of a novel synthetic inhibitor, N-[3-[4-[4-(amidinophenoxy)carbonyl]phenyl]-2-methyl-2-propenoyl]- N-allylglycine methanesulfonate, to bovine trypsin, revealed by the crystal structure of the complex.
Odagaki, Y., Nakai, H., Senokuchi, K., Kawamura, M., Hamanaka, N., Nakamura, M., Tomoo, K., Ishida, T.(1995) Biochemistry 34: 12849-12853
- PubMed: 7548040
- DOI: https://doi.org/10.1021/bi00039a046
- Primary Citation of Related Structures:
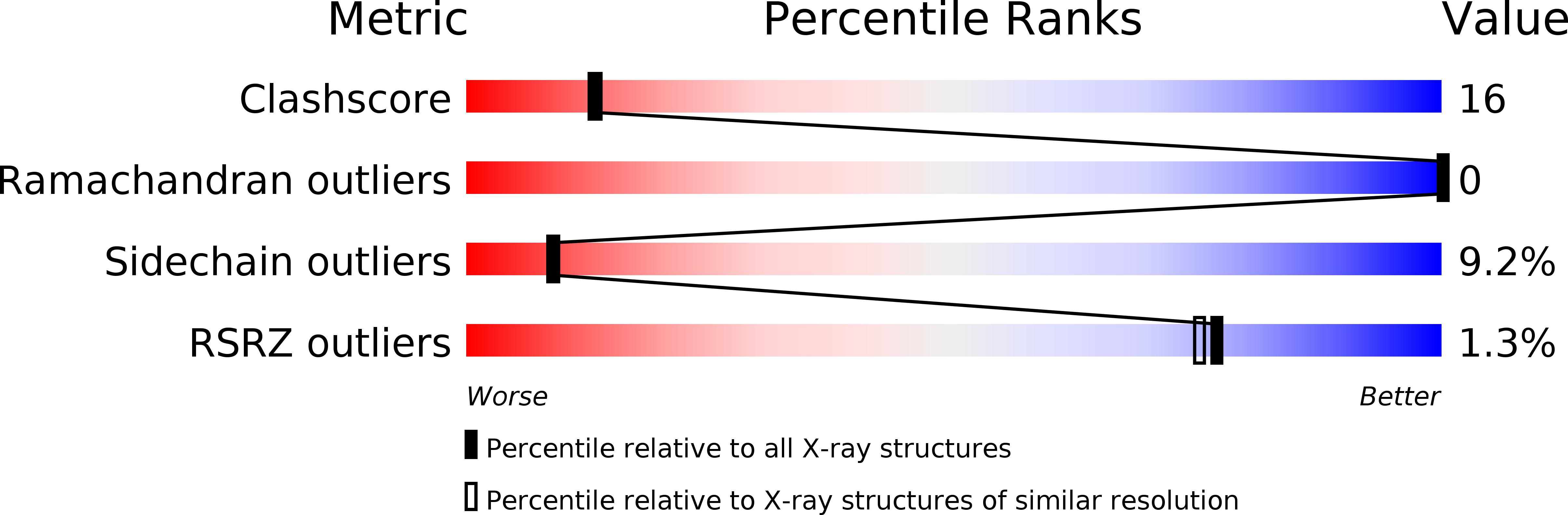
1BTP - PubMed Abstract:
Trypsin and N-[3-[4-[4-(amidinophenoxy)carbonyl]phenyl]-2-methyl-2-propenoyl]- N-allylglycine methanesulfonate (1), a newly designed and orally active synthetic trypsin inhibitor, were cocrystallized. The space group of the crystal is P2(1)2(1)2(1) with cell constants a = 63.74 A, b = 63.08 A, and c = 69.38 A, which is nearly identical to that of the orthorhombic crystal of guanidinobenzoyltrypsin. The structure was refined to a crystallographic residual R = 0.176. The refined model of the 1-trypsin complex provides the structural basis for the reaction mechanism of 1. On the basis of the present X-ray results, it is proposed that the potent inhibitory activity of 1 is mainly due to the formation of an acylated trypsin through an "inverse substrate mechanism" and its low rate of deacylation.
Organizational Affiliation:
Minase Research Institute, Ono Pharmaceutical Co., Ltd., Osaka, Japan.