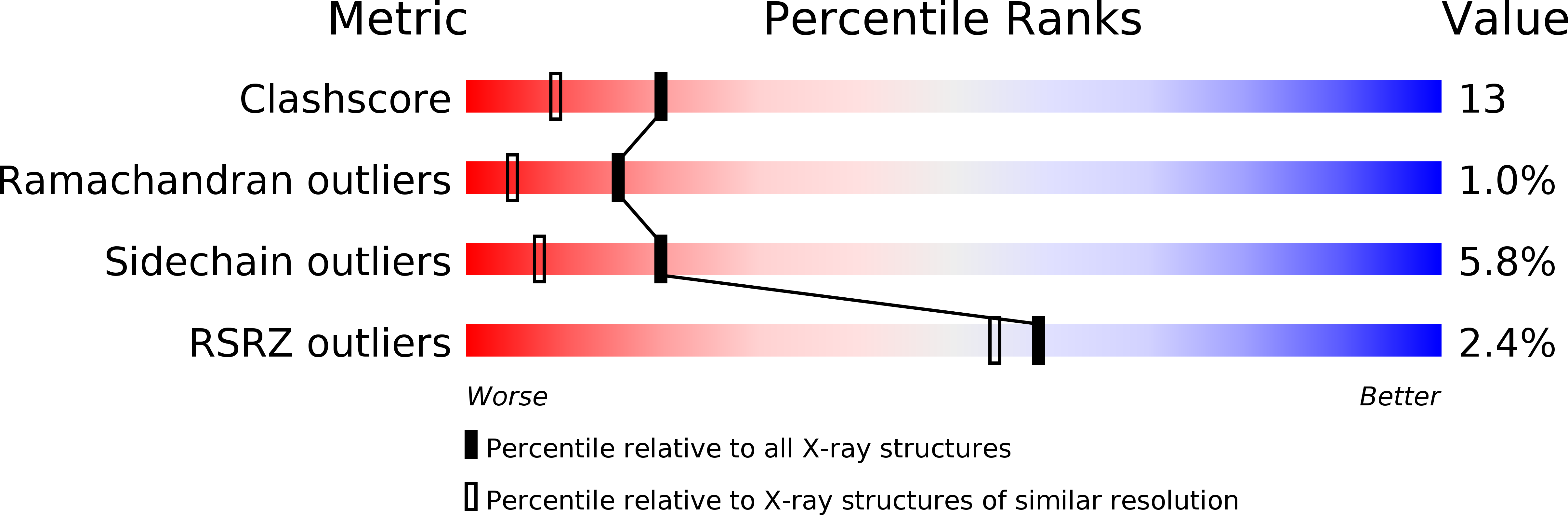
Native-like in vivo folding of a circularly permuted jellyroll protein shown by crystal structure analysis.
Hahn, M., Piotukh, K., Borriss, R., Heinemann, U.(1994) Proc Natl Acad Sci U S A 91: 10417-10421
- PubMed: 7937966
- DOI: https://doi.org/10.1073/pnas.91.22.10417
- Primary Citation of Related Structures:
1CPM, 1CPN - PubMed Abstract:
A jellyroll beta-sandwich protein, the Bacillus beta-glucanase H(A16-M), is used to probe the role of N-terminal peptide regions in protein folding in vivo. A gene encoding H(A16-M) is rearranged to place residues 1-58 of the protein behind a signal peptide and residues 59-214. The rearranged gene is expressed in Escherichia coli. The resultant circularly permuted protein, cpA16M-59, is secreted into the periplasm, correctly processed, and folded into a stable and active enzyme. Crystal structure analysis at 2.0-A resolution, R = 15.3%, shows cpA16M-59 to have a three-dimensional structure nearly identical with that of the parent beta-glucanase. An analogous experiment based on the wild-type Bacillus macerans beta-glucanase, giving rise to the circularly permuted variant cpMAC-57, yields the same results. Folding of these proteins, therefore, is not a vectorial process depending on the conformation adopted by their native N-terminal oligopeptides after ribosomal synthesis and translocation through the cytoplasmic membrane.
Organizational Affiliation:
Max-Delbrück-Centrum für Molekulare Medizin, Berlin, Germany.