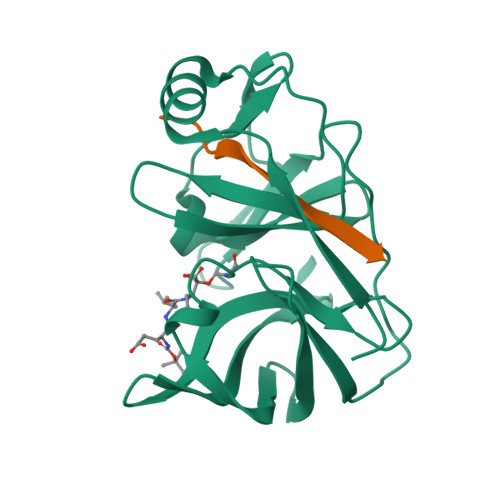
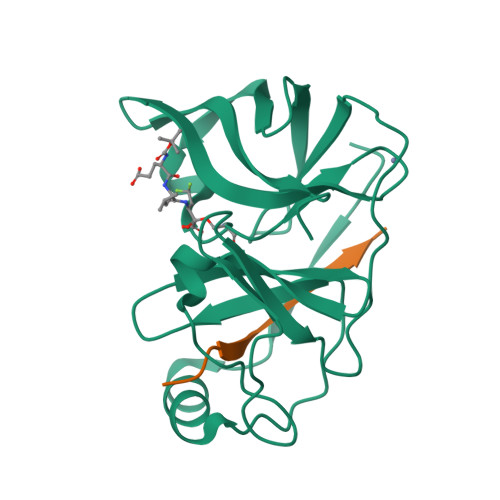
Inhibition of the Hepatitis C Virus Ns3/4A Protease the Crystal Structures of Two Protease-Inhibitor Complexes
Di Marco, S., Rizzi, M., Volpari, C., Walsh, M., Narjes, F., Colarusso, S., De Francesco, R., Matassa, V.G., Sollazzo, M.(2000) J Biol Chem 275: 7152
- PubMed: 10702283
- DOI: https://doi.org/10.1074/jbc.275.10.7152
- Primary Citation of Related Structures:
1DXP, 1DY8, 1DY9 - PubMed Abstract:
The hepatitis C virus NS3 protein contains a serine protease domain with a chymotrypsin-like fold, which is a target for development of therapeutics. We report the crystal structures of this domain complexed with NS4A cofactor and with two potent, reversible covalent inhibitors spanning the P1-P4 residues. Both inhibitors bind in an extended backbone conformation, forming an anti-parallel beta-sheet with one enzyme beta-strand. The P1 residue contributes most to the binding energy, whereas P2-P4 side chains are partially solvent exposed. The structures do not show notable rearrangements of the active site upon inhibitor binding. These results are significant for the development of antivirals.
Organizational Affiliation:
Biotechnology, Via Pontina Km 30.600, 00040 Pomezia, Rome, Italy.