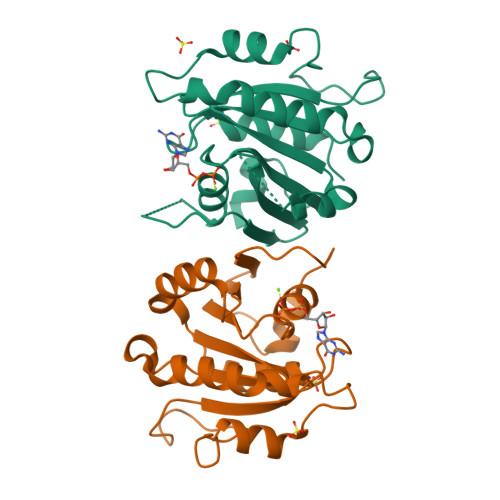
Crystal structure of Sar1-GDP at 1.7 A resolution and the role of the NH2 terminus in ER export.
Huang, M., Weissman, J.T., Beraud-Dufour, S., Luan, P., Wang, C., Chen, W., Aridor, M., Wilson, I.A., Balch, W.E.(2001) J Cell Biol 155: 937-948
- PubMed: 11739406
- DOI: https://doi.org/10.1083/jcb.200106039
- Primary Citation of Related Structures:
1F6B - PubMed Abstract:
The Sar1 GTPase is an essential component of COPII vesicle coats involved in export of cargo from the ER. We report the 1.7-A structure of Sar1 and find that consistent with the sequence divergence of Sar1 from Arf family GTPases, Sar1 is structurally distinct. In particular, we show that the Sar1 NH2 terminus contains two regions: an NH2-terminal extension containing an evolutionary conserved hydrophobic motif that facilitates membrane recruitment and activation by the mammalian Sec12 guanine nucleotide exchange factor, and an alpha1' amphipathic helix that contributes to interaction with the Sec23/24 complex that is responsible for cargo selection during ER export. We propose that the hydrophobic Sar1 NH2-terminal activation/recruitment motif, in conjunction with the alpha1' helix, mediates the initial steps in COPII coat assembly for export from the ER.
Organizational Affiliation:
Department of Cell Biology, The Scripps Research Institute, La Jolla, CA 92130, USA.