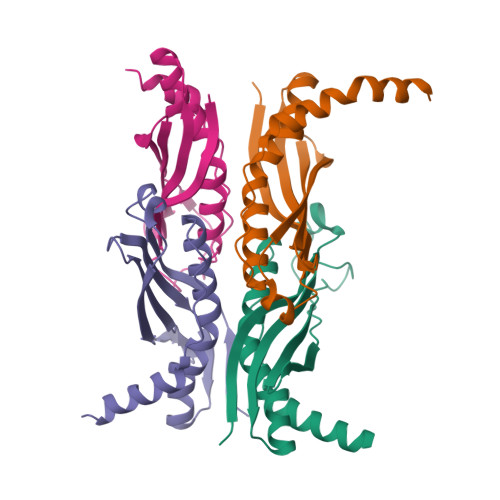
Crystal structure of the bacterial protein export chaperone secB.
Xu, Z., Knafels, J.D., Yoshino, K.(2000) Nat Struct Biol 7: 1172-1177
- PubMed: 11101901
- DOI: https://doi.org/10.1038/82040
- Primary Citation of Related Structures:
1FX3 - PubMed Abstract:
SecB is a bacterial molecular chaperone involved in mediating translocation of newly synthesized polypeptides across the cytoplasmic membrane of bacteria. The crystal structure of SecB from Haemophilus influenzae shows that the molecule is a tetramer organized as a dimer of dimers. Two long channels run along the side of the molecule. These are bounded by flexible loops and lined with conserved hydrophobic amino acids, which define a suitable environment for binding non-native polypeptides. The structure also reveals an acidic region on the top surface of the molecule, several residues of which have been implicated in binding to SecA, its downstream target.
Organizational Affiliation:
Department of Biological Chemistry, The University of Michigan Medical School, 1301 E. Catherine Road, Ann Arbor, Michigan 48109, USA. zhaohui@umich.edu